Portable filter-based microdevice for detection and characterization of circulating tumor cells
- PMID: 20876796
- PMCID: PMC2955786
- DOI: 10.1158/1078-0432.CCR-10-1105
Portable filter-based microdevice for detection and characterization of circulating tumor cells
Abstract
Purpose: Sensitive detection and characterization of circulating tumor cells (CTC) could revolutionize the approach to patients with early-stage and metastatic cancer. The current methodologies have significant limitations, including limited capture efficiency and ability to characterize captured cells. Here, we report the development of a novel parylene membrane filter-based portable microdevice for size-based isolation with high recovery rate and direct on-chip characterization of captured CTC from human peripheral blood.
Experimental design: We evaluated the sensitivity and efficiency of CTC capture in a model system using blood samples from healthy donors spiked with tumor cell lines. Fifty-nine model system samples were tested to determine the recovery rate of the microdevice. Moreover, 10 model system samples and 57 blood samples from cancer patients were subjected to both membrane microfilter device and CellSearch platform enumeration for direct comparison.
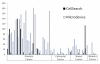
Results: Using the model system, the microdevice achieved >90% recovery with probability of 95% recovering at least one cell when five are seeded in 7.5 mL of blood. CTCs were identified in 51 of 57 patients using the microdevice, compared with only 26 patients with the CellSearch method. When CTCs were detected by both methods, greater numbers were recovered by the microfilter device in all but five patients.
Conclusions: This filter-based microdevice is both a capture and analysis platform, capable of multiplexed imaging and genetic analysis. The microdevice presented here has the potential to enable routine CTC analysis in the clinical setting for the effective management of cancer patients.
©2010 AACR.
Figures


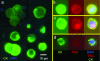
PBS only (square);
PBS with 50k LNCaP cells (circle);
PBS with 50k LNCaP cells fixed in 1% acetone (triangle);
50% human blood (triangle);
50% human blood with 25k LNCaP cells (square);
50% blood fixed in 1% NBF (triangle);
50% blood and 25k LNCaP cells fixed in 1% NBF (triangle);
50% blood and 25k LNCaP cells fixed in 2% NBF (hexagon);
50% blood and 25k LNCaP cells fixed in 5% NBF. Dash line part of curve 9 was caused by severe blocking of filter so that the filtration could not be completed (star);
100% human blood (pentagon).
Comment in
-
Reporting the capture efficiency of a filter-based microdevice: a CTC is not a CTC unless it is CD45 negative--letter.Clin Cancer Res. 2011 May 1;17(9):3048-9; author reply 3050. doi: 10.1158/1078-0432.CCR-10-3234. Clin Cancer Res. 2011. PMID: 21536548 No abstract available.
References
-
- Lugo TG, Braun S, Cote RJ, Pantel K, Rusch V. Detection and measurement of occult disease for the prognosis of solid tumors. J Clin Oncol. 2003;21:2609–15. - PubMed
-
- Schabel FM., Jr Rationale for adjuvant chemotherapy. Cancer. 1977;39:2875–82. - PubMed
-
- Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009 - PubMed
-
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

