Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy
- PMID: 20876851
- PMCID: PMC3031380
- DOI: 10.1182/blood-2010-06-288001
Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy
Abstract
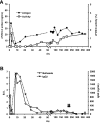
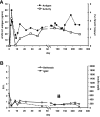
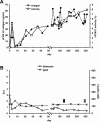
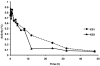
Inhibitory antibodies to factor VIII (FVIII) are a major complication in the treatment of hemophilia A, affecting approximately 20% to 30% of patients. Current treatment for inhibitors is based on long-term, daily injections of large amounts of FVIII protein. Liver-directed gene therapy has been used to induce antigen-specific tolerance, but there are no data in hemophilic animals with pre-existing inhibitors. To determine whether sustained endogenous expression of FVIII could eradicate inhibitors, we injected adeno-associated viral vectors encoding canine FVIII (cFVIII) in 2 strains of inhibitor hemophilia A dogs. In 3 dogs, a transient increase in inhibitor titers (up to 7 Bethesda Units [BU]) at 2 weeks was followed by continuous decline to complete disappearance within 4-5 weeks. Subsequently, an increase in cFVIII levels (1.5%-8%), a shortening of clotting times, and a reduction (> 90%) of bleeding episodes were observed. Immune tolerance was confirmed by lack of antibody formation after repeated challenges with cFVIII protein and normal protein half-life. A fourth dog exhibited a strong early anamnestic response (216 BU), with slow decline to 0.8 BU and cFVIII antigen detection by 18 months after vector delivery. These data suggest that liver gene therapy has the potential to eradicate inhibitors and could improve the outcomes of hemophilia A patients.
Figures


Similar articles
-
Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A.Blood. 2004 Feb 1;103(3):804-10. doi: 10.1182/blood-2003-05-1426. Epub 2003 Sep 25. Blood. 2004. PMID: 14512318
-
Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene.Thromb Res. 2006;118(5):627-35. doi: 10.1016/j.thromres.2005.11.006. Epub 2005 Dec 20. Thromb Res. 2006. PMID: 16371232
-
Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs.Blood. 2006 Jul 1;108(1):107-15. doi: 10.1182/blood-2005-12-5115. Epub 2006 Mar 7. Blood. 2006. PMID: 16522813
-
Translational Potential of Immune Tolerance Induction by AAV Liver-Directed Factor VIII Gene Therapy for Hemophilia A.Front Immunol. 2020 Apr 28;11:618. doi: 10.3389/fimmu.2020.00618. eCollection 2020. Front Immunol. 2020. PMID: 32425925 Free PMC article. Review.
-
The Immune Response to the fVIII Gene Therapy in Preclinical Models.Front Immunol. 2020 Apr 15;11:494. doi: 10.3389/fimmu.2020.00494. eCollection 2020. Front Immunol. 2020. PMID: 32351497 Free PMC article. Review.
Cited by
-
Pre-clinical evaluation of an enhanced-function factor VIII variant for durable hemophilia A gene therapy in male mice.Nat Commun. 2024 Aug 21;15(1):7193. doi: 10.1038/s41467-024-51296-8. Nat Commun. 2024. PMID: 39168991 Free PMC article.
-
Gene therapy for immune tolerance induction in hemophilia with inhibitors.J Thromb Haemost. 2016 Jun;14(6):1121-34. doi: 10.1111/jth.13331. Epub 2016 May 14. J Thromb Haemost. 2016. PMID: 27061380 Free PMC article. Review.
-
Lessons Learned from Animal Models of Inherited Bleeding Disorders.Hematol Educ. 2014 Jun;8(1):39-46. Hematol Educ. 2014. PMID: 26052366 Free PMC article.
-
Gene Therapy for Hemophilia: Facts and Quandaries in the 21st Century.Mediterr J Hematol Infect Dis. 2020 Sep 1;12(1):e2020069. doi: 10.4084/MJHID.2020.069. eCollection 2020. Mediterr J Hematol Infect Dis. 2020. PMID: 32952980 Free PMC article. Review.
-
Hemophilia a patients with inhibitors: Mechanistic insights and novel therapeutic implications.Front Immunol. 2022 Dec 8;13:1019275. doi: 10.3389/fimmu.2022.1019275. eCollection 2022. Front Immunol. 2022. PMID: 36569839 Free PMC article. Review.
References
-
- Roberts HR, Hoffman M. Hemophilia A and hemophilia B. In: Beutler E, Coller B, Lichtman M, Kipps T, Seligsohn U, editors. Hematology. 6th ed. New York, NY: McGraw-Hill; 2001. pp. 1639–1657.
-
- Dimichele D. Immune tolerance therapy for factor VIII inhibitors: moving from empiricism to an evidence-based approach. J Thromb Haemost. 2007;5(suppl 1):143–150. - PubMed
-
- Eckhardt CL, Menke LA, van Ommen CH, et al. Intensive peri-operative use of factor VIII and the Arg593—>Cys mutation are risk factors for inhibitor development in mild/moderate hemophilia A. J Thromb Haemost. 2009;7(6):930–937. - PubMed
-
- Mauser-Bunschoten EP, Fransen Van De Putte DE, Schutgens RE. Co-morbidity in the ageing haemophilia patient: the down side of increased life expectancy. Haemophilia. 2009;15(4):853–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

