Phase 1/2 trial of total marrow and lymph node irradiation to augment reduced-intensity transplantation for advanced hematologic malignancies
- PMID: 20876852
- PMCID: PMC3037752
- DOI: 10.1182/blood-2010-06-288357
Phase 1/2 trial of total marrow and lymph node irradiation to augment reduced-intensity transplantation for advanced hematologic malignancies
Abstract
This phase 1/2 study assessed the augmentation of reduced-intensity conditioning (RIC) with total marrow and lymph node irradiation (TMLI), for peripheral blood stem cell transplantation, in patients with advanced hematologic disease. The regimen consisted of fludarabine 25 mg/m(2) per day for 5 days, melphalan 140 mg/m(2) for one day, and TMLI radiation at 150 cGy/fraction in 8 fractions over 4 days. Eligible patients were over 50 years old and/or had compromised organ function. Median age of the 33 evaluable patients was 55.2 years. Eighteen events of nonhematologic grade III or higher toxicities occurred in 9 patients. Day 30 and day 100 mortalities were 3% and 15%, respectively. Patients achieved myeloid and platelet engraftment at a median of 14 days after transplantation. Long-term toxicities occurred in 2 patients: hypokalemia and tremor, both grade III, on days 370 and 361 after transplantation. Fourteen patients died, 7 of relapse-related causes and 7 of non-relapse-related causes. With a median follow-up for living patients of 14.7 months, 1-year overall survival, event-free survival, and non-relapse-related mortality were 75%, 65%, and 19%, respectively. Addition of TMLI to RIC is feasible and safe and could be offered to patients with advanced hematologic malignancies who might not otherwise be candidates for RIC.
Figures
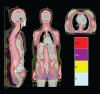
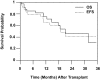
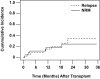
Comment in
-
TMLI: a better TBI or more of the same?Blood. 2011 Jan 6;117(1):9. doi: 10.1182/blood-2010-10-312132. Blood. 2011. PMID: 21212291 No abstract available.
References
-
- Blaise D, Maraninchi D, Michallet M, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97(11):3669–3671. - PubMed
-
- Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83(9):2723–2730. - PubMed
-
- Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32(6):543–548. - PubMed
-
- Davies SM, Ramsay NK, Klein JP, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18(2):340–347. - PubMed
-
- Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867–1871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

