In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice
- PMID: 20876972
- PMCID: PMC4005785
- DOI: 10.1088/0031-9155/55/20/007
In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice
Abstract
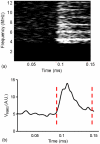
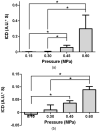
The in vivo cavitation response associated with blood-brain barrier (BBB) opening as induced by transcranial focused ultrasound (FUS) in conjunction with microbubbles was studied in order to better identify the underlying mechanism in its noninvasive application. A cylindrically focused hydrophone, confocal with the FUS transducer, was used as a passive cavitation detector (PCD) to identify the threshold of inertial cavitation (IC) in the presence of Definity® microbubbles (mean diameter range: 1.1-3.3 µm, Lantheus Medical Imaging, MA, USA). A vessel phantom was first used to determine the reliability of the PCD prior to in vivo use. A cerebral blood vessel was simulated by generating a cylindrical channel of 610 µm in diameter inside a polyacrylamide gel and by saturating its volume with microbubbles. The microbubbles were sonicated through an excised mouse skull. Second, the same PCD setup was employed for in vivo noninvasive (i.e. transdermal and transcranial) cavitation detection during BBB opening. After the intravenous administration of Definity® microbubbles, pulsed FUS was applied (frequency: 1.525 or 1.5 MHz, peak-rarefactional pressure: 0.15-0.60 MPa, duty cycle: 20%, PRF: 10 Hz, duration: 1 min with a 30 s interval) to the right hippocampus of twenty-six (n = 26) mice in vivo through intact scalp and skull. T1 and T2-weighted MR images were used to verify the BBB opening. A spectrogram was generated at each pressure in order to detect the IC onset and duration. The threshold of BBB opening was found to be at a 0.30 MPa peak-rarefactional pressure in vivo. Both the phantom and in vivo studies indicated that the IC pressure threshold had a peak-rarefactional amplitude of 0.45 MPa. This indicated that BBB opening may not require IC at or near the threshold. Histological analysis showed that BBB opening could be induced without any cellular damage at 0.30 and 0.45 MPa. In conclusion, the cavitation response could be detected without craniotomy in mice and IC may not be required for BBB opening at relatively low pressures.
Figures






References
-
- Caskey CF, Stieger SM, Qin S, Dayton PA, Ferrara KW. Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall. J. Acoust. Soc. Am. 2007;122:1191–200. - PubMed
-
- Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood–brain barrier using focused ultrasound. Phys. Med. Biol. 2007a;52:5509–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
