Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation
- PMID: 20877012
- PMCID: PMC2947225
- DOI: 10.1172/JCI42528
Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation
Abstract
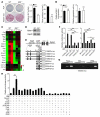
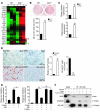
Aging leads to the disruption of the homeostatic balance of multiple biological systems. In bone marrow multipotent mesenchymal cells undergo differentiation into various anchorage-dependent cell types, including osteoblasts and adipocytes. With age as well as with treatment of antidiabetic drugs such as thiazolidinediones, mesenchymal cells favor differentiation into adipocytes, resulting in an increased number of adipocytes and a decreased number of osteoblasts, causing osteoporosis. The mechanism behind this differentiation switch is unknown. Here we show an age-related decrease in the expression of Maf in mouse mesenchymal cells, which regulated mesenchymal cell bifurcation into osteoblasts and adipocytes by cooperating with the osteogenic transcription factor Runx2 and inhibiting the expression of the adipogenic transcription factor Pparg. The crucial role of Maf in both osteogenesis and adipogenesis was underscored by in vivo observations of delayed bone formation in perinatal Maf(-/-) mice and an accelerated formation of fatty marrow associated with bone loss in aged Maf(+/-) mice. This study identifies a transcriptional mechanism for an age-related switch in cell fate determination and may provide a molecular basis for novel therapeutic strategies against age-related bone diseases.
Figures

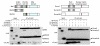


Comment in
-
c-Maf and you won't see fat.J Clin Invest. 2010 Oct;120(10):3440-2. doi: 10.1172/JCI44786. Epub 2010 Sep 27. J Clin Invest. 2010. PMID: 20877008 Free PMC article.
References
-
- Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

