Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models
- PMID: 20877209
- PMCID: PMC3126862
- DOI: 10.3390/molecules15096050
Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models
Abstract
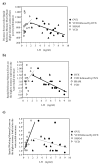
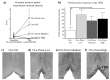
Cognitive function is multidimensional and complex, and research in multiple species indicates it is considerably impacted by age and gonadal hormone milieu. One domain of cognitive function particularly susceptible to age-related decrements is spatial memory. Gonadal hormones can alter spatial memory, and they are potent modulators of brain microstructure and function in many of the same brain areas affected by aging. In this paper, we review decades of animal and human literature to support a tertiary model representing interactions between gonadal hormones, spatial cognition and age given that: 1) gonadal hormones change with age, 2) age impacts spatial learning and memory, and 3) gonadal hormones impact spatial learning and memory. While much has been discovered regarding these individual tenets, the compass for future aging research points toward clarifying the interactions that exist between these three points, and understanding mediating variables. Indeed, identifying and aligning the various components of the complex interactions between these tenets, including evaluations using basic science, systems, and clinical perspectives, is the optimal approach to attempt to converge the many findings that may currently appear contradictory. In fact, as discoveries are being made it is becoming clear that the findings across studies that appear contradictory are not contradictory at all. Rather, there are mediating variables that are influencing outcome and affecting the extent, and even the direction, of the effects that gonadal hormones have on cognition during aging. These mediating variables are just starting to be understood. By aligning basic scientific discoveries with clinical interpretations, we can maximize the opportunities for discoveries and subsequent interventions to allow individuals to "optimize their aging" and find their own map to cognitive health as aging ensues.
Figures







Similar articles
-
Seasonal variation in gonadal hormones, spatial cognition, and hippocampal attributes: More questions than answers.Horm Behav. 2022 May;141:105151. doi: 10.1016/j.yhbeh.2022.105151. Epub 2022 Mar 14. Horm Behav. 2022. PMID: 35299119 Review.
-
The endocrine-brain-aging triad where many paths meet: female reproductive hormone changes at midlife and their influence on circuits important for learning and memory.Exp Gerontol. 2017 Aug;94:14-23. doi: 10.1016/j.exger.2016.12.011. Epub 2016 Dec 13. Exp Gerontol. 2017. PMID: 27979770 Free PMC article.
-
Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline.Prog Brain Res. 2010;182:31-76. doi: 10.1016/S0079-6123(10)82002-3. Prog Brain Res. 2010. PMID: 20541660 Review.
-
Sex differences in sleep and sleep loss-induced cognitive deficits: The influence of gonadal hormones.Horm Behav. 2019 Feb;108:50-61. doi: 10.1016/j.yhbeh.2018.12.013. Epub 2019 Jan 15. Horm Behav. 2019. PMID: 30597139 Review.
-
Translational cognitive endocrinology: designing rodent experiments with the goal to ultimately enhance cognitive health in women.Brain Res. 2013 Jun 13;1514:50-62. doi: 10.1016/j.brainres.2013.01.020. Epub 2013 Feb 4. Brain Res. 2013. PMID: 23391594 Free PMC article. Review.
Cited by
-
The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders.Sci Transl Med. 2015 Jul 22;7(297):297ra113. doi: 10.1126/scitranslmed.aab1290. Sci Transl Med. 2015. PMID: 26203081 Free PMC article.
-
Altered brain rhythms and behaviour in the accelerated ovarian failure mouse model of human menopause.Brain Commun. 2022 Jun 22;4(4):fcac166. doi: 10.1093/braincomms/fcac166. eCollection 2022. Brain Commun. 2022. PMID: 35794872 Free PMC article.
-
Mente Activa® Improves Impaired Spatial Memory in Aging Rats.J Nutr Health Aging. 2015 Oct;19(8):819-27. doi: 10.1007/s12603-015-0546-4. J Nutr Health Aging. 2015. PMID: 26412286
-
Age Impacts the Burden That Reference Memory Imparts on an Increasing Working Memory Load and Modifies Relationships With Cholinergic Activity.Front Behav Neurosci. 2021 Feb 10;15:610078. doi: 10.3389/fnbeh.2021.610078. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33643006 Free PMC article.
-
Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity.Psychoneuroendocrinology. 2015 Apr;54:1-13. doi: 10.1016/j.psyneuen.2015.01.002. Epub 2015 Jan 12. Psychoneuroendocrinology. 2015. PMID: 25679306 Free PMC article.
References
-
- U.S. Census Bureau [(accessed online on April 21, 2007.)];Population Information. http://www.census.gov/ipc/www/world.html/.
-
- Tulving E., Craik F.I. The Oxford Handbook of Memory. Oxford University Press; New York, NY, USA: 2000.
-
- Kausler D.H. Learning and Memory in Normal Aging. Academic Press; New York, NY, USA: 1994.
-
- Balota D.A., Dolan P.O., Duchek J.M. The Oxford Handbook of Memory. Oxford University Press; New York, NY, USA: 2000. Memory changes in healthy young and older adults; pp. 395–410.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

