Identification of a small molecule activator of novel PKCs for promoting glucose-dependent insulin secretion
- PMID: 20877311
- PMCID: PMC3164238
- DOI: 10.1038/cr.2010.137
Identification of a small molecule activator of novel PKCs for promoting glucose-dependent insulin secretion
Abstract
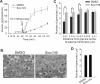
Using an image-based screen for small molecules that can affect Golgi morphology, we identify a small molecule, Sioc145, which can enlarge the Golgi compartments and promote protein secretion. More importantly, Sioc145 potentiates insulin secretion in a glucose-dependent manner. We show that Sioc145 selectively activates novel protein kinase Cs (nPKCs; δ and ɛ) but not conventional PKCs (cPKCs; α, βI and βII) in INS-1E insulinoma cells. In contrast, PMA, a non-selective activator of cPKCs and nPKCs, promotes insulin secretion independent of glucose concentrations. Furthermore, we demonstrate that Sioc145 and PMA show differential abilities in depolarizing the cell membrane, and suggest that Sioc145 promotes insulin secretion in the amplifying pathway downstream of K(ATP) channels. In pancreatic islets, the treatment with Sioc145 enhances the second phase of insulin secretion. Increased insulin granules close to the plasma membrane are observed after Sioc145 treatment. Finally, the administration of Sioc145 to diabetic GK rats increases their serum insulin levels and improves glucose tolerance. Collectively, our studies identify Sioc145 as a novel glucose-dependent insulinotropic compound via selectively activating nPKCs.
Figures





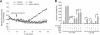
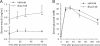
References
-
- Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18:451–463. - PubMed
-
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. - PubMed
-
- Henquin JC. Pathways in β-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53 Suppl 3:S48–58. - PubMed
-
- Knutson KL, Hoenig M. Identification and subcellular characterization of protein kinase-C isoforms in insulinoma β-cells and whole islets. Endocrinology. 1994;135:881–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

