Shedding light on avian influenza H4N6 infection in mallards: modes of transmission and implications for surveillance
- PMID: 20877466
- PMCID: PMC2942899
- DOI: 10.1371/journal.pone.0012851
Shedding light on avian influenza H4N6 infection in mallards: modes of transmission and implications for surveillance
Abstract
Background: Wild mallards (Anas platyrhychos) are considered one of the primary reservoir species for avian influenza viruses (AIV). Because AIV circulating in wild birds pose an indirect threat to agriculture and human health, understanding the ecology of AIV and developing risk assessments and surveillance systems for prevention of disease is critical.
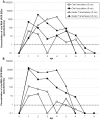
Methodology/principal findings: In this study, mallards were experimentally infected with an H4N6 subtype of AIV by oral inoculation or contact with an H4N6 contaminated water source. Cloacal swabs, oropharyngeal swabs, fecal samples, and water samples were collected daily and tested by real-time RT-PCR (RRT-PCR) for estimation of viral shedding. Fecal samples had significantly higher virus concentrations than oropharyngeal or cloacal swabs and 6 month old ducks shed significantly more viral RNA than 3 month old ducks regardless of sample type. Use of a water source contaminated by AIV infected mallards, was sufficient to transmit virus to naïve mallards, which shed AIV at higher or similar levels as orally-inoculated ducks.
Conclusions: Bodies of water could serve as a transmission pathway for AIV in waterfowl. For AIV surveillance purposes, water samples and fecal samples appear to be excellent alternatives or additions to cloacal and oropharyngeal swabbing. Furthermore, duck age (even within hatch-year birds) may be important when interpreting viral shedding results from experimental infections or surveillance. Differential shedding among hatch-year mallards could affect prevalence estimates, modeling of AIV spread, and subsequent risk assessments.
Conflict of interest statement
Figures
Similar articles
-
Active virological surveillance in backyard ducks in Bangladesh: detection of avian influenza and gammacoronaviruses.Avian Pathol. 2020 Aug;49(4):361-368. doi: 10.1080/03079457.2020.1753654. Epub 2020 May 18. Avian Pathol. 2020. PMID: 32271094
-
Variation in viral shedding patterns between different wild bird species infected experimentally with low-pathogenicity avian influenza viruses that originated from wild birds.Avian Pathol. 2011 Apr;40(2):119-24. doi: 10.1080/03079457.2010.540002. Avian Pathol. 2011. PMID: 21500030
-
Intestinal excretion of a wild bird-origin H3N8 low pathogenic avian influenza virus in mallards (Anas Platyrhynchos).J Wildl Dis. 2012 Oct;48(4):991-8. doi: 10.7589/2011-09-280. J Wildl Dis. 2012. PMID: 23060500 Free PMC article.
-
The effect of age on avian influenza viral shedding in mallards (Anas platyrhynchos).Avian Dis. 2010 Mar;54(1 Suppl):581-5. doi: 10.1637/8692-031309-ResNote.1. Avian Dis. 2010. PMID: 20521698 Free PMC article.
-
Failure of productive infection of Mallards (Anas platyrhynchos) with H16 subtype of avian influenza viruses.Influenza Other Respir Viruses. 2014 Nov;8(6):613-6. doi: 10.1111/irv.12275. Epub 2014 Sep 10. Influenza Other Respir Viruses. 2014. PMID: 25205059 Free PMC article.
Cited by
-
Environmental and demographic determinants of avian influenza viruses in waterfowl across the contiguous United States.PLoS One. 2012;7(3):e32729. doi: 10.1371/journal.pone.0032729. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427870 Free PMC article.
-
Understanding the ecological drivers of avian influenza virus infection in wildfowl: a continental-scale study across Africa.Proc Biol Sci. 2012 Mar 22;279(1731):1131-41. doi: 10.1098/rspb.2011.1417. Epub 2011 Sep 14. Proc Biol Sci. 2012. PMID: 21920984 Free PMC article.
-
Does influenza A virus infection affect movement behaviour during stopover in its wild reservoir host?R Soc Open Sci. 2016 Feb 10;3(2):150633. doi: 10.1098/rsos.150633. eCollection 2016 Feb. R Soc Open Sci. 2016. PMID: 26998334 Free PMC article.
-
Improving risk assessment of the emergence of novel influenza A viruses by incorporating environmental surveillance.Philos Trans R Soc Lond B Biol Sci. 2019 Sep 30;374(1782):20180346. doi: 10.1098/rstb.2018.0346. Epub 2019 Aug 12. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31401963 Free PMC article.
-
Low-pathogenic avian influenza viruses in wild house mice.PLoS One. 2012;7(6):e39206. doi: 10.1371/journal.pone.0039206. Epub 2012 Jun 15. PLoS One. 2012. PMID: 22720076 Free PMC article.
References
-
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus ADME, et al. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. - PubMed
-
- Halvorson DA, Frame DD, Friendshuh KAJ, Shaw DP. Outbreaks of low pathogenicity avian influenza in U.S.A. Avian Dis. 2003;47, Special Issue, Fourth International Symposium on Avian Influenza, 1997 Proceedings:36–46.
-
- Horimoto T, Kawaoka Y. Influenza: Lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

