Neural activity patterns in response to interspecific and intraspecific variation in mating calls in the túngara frog
- PMID: 20877560
- PMCID: PMC2943914
- DOI: 10.1371/journal.pone.0012898
Neural activity patterns in response to interspecific and intraspecific variation in mating calls in the túngara frog
Abstract
Background: During mate choice, individuals must classify potential mates according to species identity and relative attractiveness. In many species, females do so by evaluating variation in the signals produced by males. Male túngara frogs (Physalaemus pustulosus) can produce single note calls (whines) and multi-note calls (whine-chucks). While the whine alone is sufficient for species recognition, females greatly prefer the whine-chuck when given a choice.
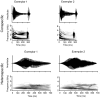
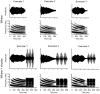
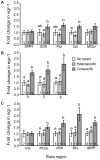
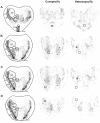
Methodology/principal findings: To better understand how the brain responds to variation in male mating signals, we mapped neural activity patterns evoked by interspecific and intraspecific variation in mating calls in túngara frogs by measuring expression of egr-1. We predicted that egr-1 responses to conspecific calls would identify brain regions that are potentially important for species recognition and that at least some of those brain regions would vary in their egr-1 responses to mating calls that vary in attractiveness. We measured egr-1 in the auditory brainstem and its forebrain targets and found that conspecific whine-chucks elicited greater egr-1 expression than heterospecific whines in all but three regions. We found no evidence that preferred whine-chuck calls elicited greater egr-1 expression than conspecific whines in any of eleven brain regions examined, in contrast to predictions that mating preferences in túngara frogs emerge from greater responses in the auditory system.
Conclusions: Although selectivity for species-specific signals is apparent throughout the túngara frog brain, further studies are necessary to elucidate how neural activity patterns vary with the attractiveness of conspecific mating calls.
Conflict of interest statement
Figures


Similar articles
-
Auditory selectivity for acoustic features that confer species recognition in the tungara frog.J Exp Biol. 2011 Sep 1;214(Pt 17):2911-8. doi: 10.1242/jeb.058362. J Exp Biol. 2011. PMID: 21832134
-
Effects of estradiol on neural responses to social signals in female túngara frogs.J Exp Biol. 2015 Nov;218(Pt 22):3671-7. doi: 10.1242/jeb.127738. Epub 2015 Oct 8. J Exp Biol. 2015. PMID: 26449971
-
Treatment with arginine vasotocin alters mating calls and decreases call attractiveness in male túngara frogs.Gen Comp Endocrinol. 2010 Jan 15;165(2):221-8. doi: 10.1016/j.ygcen.2009.06.023. Epub 2009 Jul 1. Gen Comp Endocrinol. 2010. PMID: 19576218
-
Neurobiology of Female Mate Choice in Frogs: Auditory Filtering and Valuation.Integr Comp Biol. 2017 Oct 1;57(4):857-864. doi: 10.1093/icb/icx098. Integr Comp Biol. 2017. PMID: 29048536 Review.
-
Mating vocalizations of female frogs: control and evolutionary mechanisms.Brain Behav Evol. 1999;53(4):187-97. doi: 10.1159/000006594. Brain Behav Evol. 1999. PMID: 10343085 Review.
Cited by
-
Male mice song syntax depends on social contexts and influences female preferences.Front Behav Neurosci. 2015 Apr 1;9:76. doi: 10.3389/fnbeh.2015.00076. eCollection 2015. Front Behav Neurosci. 2015. PMID: 25883559 Free PMC article.
-
From uni- to multimodality: towards an integrative view on anuran communication.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014 Sep;200(9):777-87. doi: 10.1007/s00359-014-0923-1. Epub 2014 Jun 29. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014. PMID: 24973893 Free PMC article. Review.
-
Birdsong: is it music to their ears?Front Evol Neurosci. 2012 Nov 28;4:14. doi: 10.3389/fnevo.2012.00014. eCollection 2012. Front Evol Neurosci. 2012. PMID: 23226128 Free PMC article.
-
Tuned in to communication sounds: Neuronal sensitivity in the túngara frog midbrain to frequency modulated signals.PLoS One. 2022 May 19;17(5):e0268383. doi: 10.1371/journal.pone.0268383. eCollection 2022. PLoS One. 2022. PMID: 35587486 Free PMC article.
-
Social signals increase monoamine levels in the tegmentum of juvenile Mexican spadefoot toads (Spea multiplicata).J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Aug;199(8):681-91. doi: 10.1007/s00359-013-0826-6. Epub 2013 May 17. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23681220
References
-
- Arnold ML. Natural Hybridization and Evolution; In: May RM, Harvey PH, editors. Oxford: Oxford University Press; 1997. 232
-
- Gerhardt HC, Huber F. Chicago: University of Chicago Press; 2002. Acoustic communication in insects and anurans.542
-
- Andersson M. Princeton: Princeton University Press; 1994. Sexual selection.624
-
- Endler JA. Signals, signal conditions and the direction of evolution. Am Nat. 1992;139:S125–S153.
-
- Gerhardt HC. Female mate choice in treefrogs - static and dynamic acoustic criteria. Anim Behav. 1991;42:615–635.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

