Ricin toxicokinetics and its sensitive detection in mouse sera or feces using immuno-PCR
- PMID: 20877567
- PMCID: PMC2943921
- DOI: 10.1371/journal.pone.0012858
Ricin toxicokinetics and its sensitive detection in mouse sera or feces using immuno-PCR
Abstract
Background: Ricin (also called RCA-II or RCA(60)), one of the most potent toxins and documented bioweapons, is derived from castor beans of Ricinus communis. Several in vitro methods have been designed for ricin detection in complex food matrices in the event of intentional contamination. Recently, a novel Immuno-PCR (IPCR) assay was developed with a limit of detection of 10 fg/ml in a buffer matrix and about 10-1000-fold greater sensitivity than other methods in various food matrices.
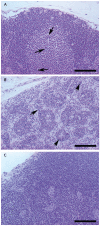
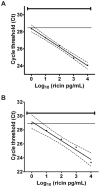
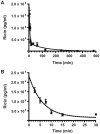
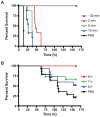
Methods and findings: In order to devise a better diagnostic test for ricin, the IPCR assay was adapted for the detection of ricin in biological samples collected from mice after intoxication. The limit of detection in both mouse sera and feces was as low as 1 pg/ml. Using the mouse intravenous (iv) model for ricin intoxication, a biphasic half-life of ricin, with a rapid t(1/2)α of 4 min and a slower t(1/2)β of 86 min were observed. The molecular biodistribution time for ricin following oral ingestion was estimated using an antibody neutralization assay. Ricin was detected in the blood stream starting at approximately 6-7 h post- oral intoxication. Whole animal histopathological analysis was performed on mice treated orally or systemically with ricin. Severe lesions were observed in the pancreas, spleen and intestinal mesenteric lymph nodes, but no severe pathology in other major organs was observed.
Conclusions: The determination of in vivo toxicokinetics and pathological effects of ricin following systemic and oral intoxication provide a better understanding of the etiology of intoxication and will help in the future design of more effective diagnostic and therapeutic methods.
Conflict of interest statement
Figures
Similar articles
-
Detection of ricin contamination in ground beef by electrochemiluminescence immunosorbent assay.Toxins (Basel). 2011 Apr;3(4):398-408. doi: 10.3390/toxins3040398. Epub 2011 Apr 4. Toxins (Basel). 2011. PMID: 22069715 Free PMC article.
-
Serial ricinine levels in serum and urine after ricin intoxication.J Anal Toxicol. 2013 Jun;37(5):313-7. doi: 10.1093/jat/bkt026. Epub 2013 Apr 16. J Anal Toxicol. 2013. PMID: 23592744
-
Detection of castor contamination by real-time polymerase chain reaction.J Agric Food Chem. 2007 Jan 24;55(2):545-50. doi: 10.1021/jf062381r. J Agric Food Chem. 2007. PMID: 17227091
-
Ricin poisoning: A review on contamination source, diagnosis, treatment, prevention and reporting of ricin poisoning.Toxicon. 2021 May;195:86-92. doi: 10.1016/j.toxicon.2021.03.004. Epub 2021 Mar 9. Toxicon. 2021. PMID: 33711365 Review.
-
Ricin Poisoning after Oral Ingestion of Castor Beans: A Case Report and Review of the Literature and Laboratory Testing.J Emerg Med. 2017 Nov;53(5):e67-e71. doi: 10.1016/j.jemermed.2017.08.023. Epub 2017 Oct 4. J Emerg Med. 2017. PMID: 28987302 Review.
Cited by
-
Medical Countermeasures against Ricin Intoxication.Toxins (Basel). 2023 Jan 20;15(2):100. doi: 10.3390/toxins15020100. Toxins (Basel). 2023. PMID: 36828415 Free PMC article. Review.
-
Characterization of Ricin and R. communis Agglutinin Reference Materials.Toxins (Basel). 2015 Nov 26;7(12):4906-34. doi: 10.3390/toxins7124856. Toxins (Basel). 2015. PMID: 26703723 Free PMC article.
-
A Self-Driven Microfluidic Chip for Ricin and Abrin Detection.Sensors (Basel). 2022 May 2;22(9):3461. doi: 10.3390/s22093461. Sensors (Basel). 2022. PMID: 35591151 Free PMC article.
-
Animal models of ricin toxicosis.Curr Top Microbiol Immunol. 2012;357:243-57. doi: 10.1007/82_2011_173. Curr Top Microbiol Immunol. 2012. PMID: 21956160 Free PMC article.
-
Ricinus communis intoxications in human and veterinary medicine-a summary of real cases.Toxins (Basel). 2011 Oct;3(10):1332-72. doi: 10.3390/toxins3101332. Epub 2011 Oct 24. Toxins (Basel). 2011. PMID: 22069699 Free PMC article. Review.
References
-
- Greenfield RA, Brown BR, Hutchins JB, Iandolo JJ, Jackson R, et al. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci. 2002;323:326–40. - PubMed
-
- Balint GA. Ricin: the toxic protein of castor oil seeds. Toxicology. 1974;2:77–102. - PubMed
-
- Franz DR, Jaax NK. Ricin Toxin. Medical Aspects of Chemical and Biological Warfare; Chapter. 1997;32:631–642.
-
- Doan LG. Ricin: mechanism of toxicity, clinical manifestations, and vaccine development. J Toxicol Clin Toxicol. 2004;42:201–208. - PubMed
-
- Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. Jama. 2005;294:2342–2351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

