Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa
- PMID: 20877623
- PMCID: PMC2943475
- DOI: 10.1371/journal.pone.0012873
Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa
Abstract
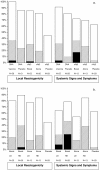
Background: We conducted a double-blind, randomized, placebo-controlled Phase I study of a recombinant replication-defective adenovirus type 5 (rAd5) vector expressing HIV-1 Gag and Pol from subtype B and Env from subtypes A, B and C, given alone or as boost following a DNA plasmid vaccine expressing the same HIV-1 proteins plus Nef, in 114 healthy HIV-uninfected African adults.
Methodology/principal findings: Volunteers were randomized to 4 groups receiving the rAd5 vaccine intramuscularly at dosage levels of 1×10(10) or 1×10(11) particle units (PU) either alone or as boost following 3 injections of the DNA vaccine given at 4 mg/dose intramuscularly by needle-free injection using Biojector® 2000. Safety and immunogenicity were evaluated for 12 months. Both vaccines were well-tolerated. Overall, 62% and 86% of vaccine recipients in the rAd5 alone and DNA prime - rAd5 boost groups, respectively, responded to the HIV-1 proteins by an interferon-gamma (IFN-γ) ELISPOT. The frequency of immune responses was independent of rAd5 dosage levels. The highest frequency of responses after rAd5 alone was detected at 6 weeks; after DNA prime - rAd5 boost, at 6 months (end of study). At baseline, neutralizing antibodies against Ad5 were present in 81% of volunteers; the distribution was similar across the 4 groups. Pre-existing immunity to Ad5 did not appear to have a significant impact on reactogenicity or immune response rates to HIV antigens by IFN-γ ELISPOT. Binding antibodies against Env were detected in up to 100% recipients of DNA prime - rAd5 boost. One volunteer acquired HIV infection after the study ended, two years after receipt of rAd5 alone.
Conclusions/significance: The HIV-1 rAd5 vaccine, either alone or as a boost following HIV-1 DNA vaccine, was well-tolerated and immunogenic in African adults. DNA priming increased the frequency and magnitude of cellular and humoral immune responses, but there was no effect of rAd5 dosage on immunogenicity endpoints.
Trial registration: ClinicalTrials.gov NCT00124007.
Conflict of interest statement
Figures






References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–4092. - PubMed
-
- Eller MA, Eller LA, Opollo MS, Ouma BJ, Oballah PO, et al. Induction of HIV-specific functional immune responses by a multiclade HIV-1 DNA vaccine candidate in healthy Ugandans. Vaccine. 2007;25:7737–7742. - PubMed

