A tonsillar PolyICLC/AT-2 SIV therapeutic vaccine maintains low viremia following antiretroviral therapy cessation
- PMID: 20877632
- PMCID: PMC2943484
- DOI: 10.1371/journal.pone.0012891
A tonsillar PolyICLC/AT-2 SIV therapeutic vaccine maintains low viremia following antiretroviral therapy cessation
Abstract
Background: HIV-infected individuals rely on antiretroviral therapy (ART) to control viral replication. Despite abundant demonstrable benefits, the multiple limitations of ART point to the potential advantages of therapeutic vaccination approaches that could provide sustained host control of viral replication after discontinuation of ART. We provide evidence from a non-human primate model that a therapeutic vaccine applied to the tonsils can maintain low viral loads after cessation of ART.
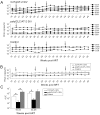
Methodology/principal findings: Animals received 40 weeks of ART initiated 9 weeks after rectal SIVmac239 infection. During ART, animals were vaccinated (or not) with AT-2 inactivated SIVmac239 using CpG-C ISS-ODN (C274) or polyICLC as adjuvants. PolyICLC/AT-2 SIV vaccinated animals maintained viral loads <3×10(3) copies/ml for up to 16 weeks post-ART, whereas the C274/AT-2 SIV vaccinated and non-vaccinated animals' viremia ranged between 1×10(4)-4×10(5) copies/ml (p<0.03). Neutralizing Ab activity in plasma was increased by polyICLC/AT-2 tonsillar vaccination under ART, compared to controls (p<0.03). Subsequent vaccination of all animals with polyICLC/AT-2 SIV in the absence of ART did not alter viral loads. Other immune parameters measured in blood and tissues were comparable between groups.
Conclusions/significance: These results provide support for the potential benefit of mucosally delivered vaccines in therapeutic immunization strategies for control of AIDS virus infection.
Conflict of interest statement
Figures


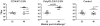
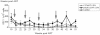
References
-
- Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. Jama. 2000;283:381–390. - PubMed
-
- Este JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antiviral Res. 2010;85:25–33. - PubMed
-
- Wensing AM, van de Vijver DA, Angarano G, Asjo B, Balotta C, et al. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis. 2005;192:958–966. - PubMed
-
- Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

