Seventy-five trials and eleven systematic reviews a day: how will we ever keep up?
- PMID: 20877712
- PMCID: PMC2943439
- DOI: 10.1371/journal.pmed.1000326
Seventy-five trials and eleven systematic reviews a day: how will we ever keep up?
Abstract
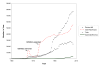
When Archie Cochrane reproached the medical profession for not having critical summaries of all randomised controlled trials, about 14 reports of trials were being published per day. There are now 75 trials, and 11 systematic reviews of trials, per day and a plateau in growth has not yet been reached. Although trials, reviews, and health technology assessments have undoubtedly had major impacts, the staple of medical literature synthesis remains the non-systematic narrative review. Only a small minority of trial reports are being analysed in up-to-date systematic reviews. Given the constraints, Archie Cochrane's vision will not be achieved without some serious changes in course. To meet the needs of patients, clinicians, and policymakers, unnecessary trials need to be reduced, and systematic reviews need to be prioritised. Streamlining and innovation in methods of systematic reviewing are necessary to enable valid answers to be found for most patient questions. Finally, clinicians and patients require open access to these important resources.
Conflict of interest statement
HB works for a health technology assessment agency.
Figures



References
-
- Cochrane AL. Medicines for the Year 2000. London: Office of Health Economics; 1979. 1931–1971: a critical review, with particular reference to the medical profession. pp. 1–11.
-
- Lind J. 1753. A treatise of the scurvy. In three parts. Containing an inquiry into the nature, causes and cure, of that disease. Together with a critical and chronological view of what has been published on the subject. Edinburgh: Printed by Sands, Murray and Cochran for A Kincaid and A Donaldson. Accessed: 26 April 2009 http://www.jameslindlibrary.org/trial_records/17th_18th_Century/lind/lin....
-
- Duncan A. Introduction. Medical and Philosophical Commentaries. Volume First, Part I. London: J Murray. 1773:6–7.
-
- Cummings MM. The National Library of Medicine. In: Warren KS, editor. Coping with the biomedical literature: A primer for the scientist and the clinician. New York: Praeger; 1981. pp. 161–173.
-
- Barron BA, Bukantz SC. The evaluation of new drugs: current Food and Drug Administration regulations and statistical aspects of clinical trials. Arch Intern Med. 1967;119:547–556. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

