S(N)Ar-based, facile synthesis of a library of benzothiaoxazepine-1,1'-dioxides
- PMID: 20879738
- PMCID: PMC3098767
- DOI: 10.1021/cc1001023
S(N)Ar-based, facile synthesis of a library of benzothiaoxazepine-1,1'-dioxides
Abstract
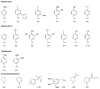
The construction of a library of benzothiaoxazepine-1,1'-dioxides utilizing a one-pot, S(N)Ar diversification-ODCT(50) scavenging protocol is reported. This protocol combines microwave irradiation to facilitate the reaction, in conjunction with a soluble ROMP-derived scavenger (ODCT) to afford the desired products in good overall purity. Utilizing this protocol, a 78-member library was successfully synthesized and submitted for biological evaluation.
Figures

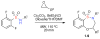


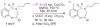

Similar articles
-
Synthesis of amino-benzothiaoxazepine-1,1-dioxides utilizing a microwave-assisted, S(N)Ar protocol.ACS Comb Sci. 2011 Nov 14;13(6):653-8. doi: 10.1021/co200076j. Epub 2011 Sep 8. ACS Comb Sci. 2011. PMID: 21902243 Free PMC article.
-
Facile (triazolyl)methylation of MACOS-derived benzofused sultams utilizing ROMP-derived OTP reagents.ACS Comb Sci. 2012 Apr 9;14(4):268-72. doi: 10.1021/co2001839. Epub 2012 Mar 5. ACS Comb Sci. 2012. PMID: 22384820 Free PMC article.
-
One-pot, three-component, domino Heck-aza-Michael approach to libraries of functionalized 1,1-dioxido-1,2-benzisothiazoline-3-acetic acids.J Comb Chem. 2009 Jul-Aug;11(4):732-8. doi: 10.1021/cc900025e. J Comb Chem. 2009. PMID: 19505109 Free PMC article.
-
Liquid-phase combinatorial library synthesis: recent advances and future perspectives.Comb Chem High Throughput Screen. 2014;17(5):417-38. doi: 10.2174/1386207316666131117172549. Comb Chem High Throughput Screen. 2014. PMID: 24237348 Review.
-
Organic selenium resin in solid phase synthesis and its application in constructing medicinally relevant small organic molecules.Mini Rev Med Chem. 2013 May 1;13(6):854-69. doi: 10.2174/1389557511313060008. Mini Rev Med Chem. 2013. PMID: 23544465 Review.
Cited by
-
Synthesis of amino-benzothiaoxazepine-1,1-dioxides utilizing a microwave-assisted, S(N)Ar protocol.ACS Comb Sci. 2011 Nov 14;13(6):653-8. doi: 10.1021/co200076j. Epub 2011 Sep 8. ACS Comb Sci. 2011. PMID: 21902243 Free PMC article.
-
Parallel microwave chemistry in silicon carbide microtiter platforms: a review.Mol Divers. 2012 Feb;16(1):5-25. doi: 10.1007/s11030-011-9346-x. Epub 2011 Nov 30. Mol Divers. 2012. PMID: 22127640 Review.
-
Application of Silica-Supported Alkylating Reagents in a One-Pot, Sequential Protocol to Diverse Benzoxathiazepine 1,1-Dioxides.ACS Comb Sci. 2016 Jul 11;18(7):387-93. doi: 10.1021/acscombsci.6b00041. Epub 2016 Jun 14. ACS Comb Sci. 2016. PMID: 27300570 Free PMC article.
-
Accessing Stereochemically Rich Sultams via Microwave-Assisted, Continuous Flow Organic Synthesis (MACOS) Scale-out.J Flow Chem. 2011 Aug;1(1):32-39. doi: 10.1556/jfchem.2011.00008. J Flow Chem. 2011. PMID: 22116791 Free PMC article.
-
Exploring chemical diversity via a modular reaction pairing strategy.Beilstein J Org Chem. 2012;8:1293-302. doi: 10.3762/bjoc.8.147. Epub 2012 Aug 15. Beilstein J Org Chem. 2012. PMID: 23019462 Free PMC article.
References
-
-
For reviews concerning ROMP reagents, see: Barrett AGM, Hopkins BT, Köbberling J. Chem Rev. 2002;102:3301–3324.. Flynn DL, Hanson PR, Berk SC, Makara GM. Curr Opin Drug Discovery Dev. 2002;5:571–579.. Harned AM, Probst DA, Hanson PR. The Use of Olefin Metathesis in Combinatorial Chemistry: Supported and Chromatography-Free Syntheses. In: Grubbs RH, editor. Handbook of Metathesis. Wiley-VCH; Weinheim, Germany: 2003. pp. 361–402.. Harned AM, Zhang M, Vedantham P, Mukherjee S, Herpel RH, Flynn DL, Hanson PR. Aldrichim Acta. 2005;38:3–16.
-
-
- Nicolaou KC, Chen JS. Chem Soc Rev. 2009;11:2993–3009. - PMC - PubMed
- Enders D, Grondal C, Hüttl MRM. Angew Chem Int Ed. 2007;46:1570–1581. - PubMed
- Tietze LF, Beifuss U. Angew Chem Int Ed Engl. 1993;32:131–163.
- Tietze LF, Brasche G, Gericke KM, editors. Wiley-VCH; Weinheim, Germany: 2006.
- Rolfe A, Young K, Hanson PR. Eur J Org Chem. 2008:5254–5262. - PMC - PubMed
-
- Zhuang L, Wai JS, Embrey MW, Fisher TE, Egbertson MS, Payne LS, Guare JP, Jr, Vacca JP, Hazuda DJ, Felock PJ, Wolfe AL, Stillmock KA, Witmer MV, Moyer G, Schleif WA, Gabryelski LJ, Leonard YM, Lynch JJ, Jr, Michelson SR, Young SD. J Med Chem. 2003;46:453–456. - PubMed
-
- Levy L. Drugs Future. 1992;17:451–454.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

