Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma
- PMID: 20879881
- PMCID: PMC3086629
- DOI: 10.1056/NEJMoa0911123
Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma
Abstract
Background: Preclinical and preliminary clinical data indicate that ch14.18, a monoclonal antibody against the tumor-associated disialoganglioside GD2, has activity against neuroblastoma and that such activity is enhanced when ch14.18 is combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-2. We conducted a study to determine whether adding ch14.18, GM-CSF, and interleukin-2 to standard isotretinoin therapy after intensive multimodal therapy would improve outcomes in high-risk neuroblastoma.
Methods: Patients with high-risk neuroblastoma who had a response to induction therapy and stem-cell transplantation were randomly assigned, in a 1:1 ratio, to receive standard therapy (six cycles of isotretinoin) or immunotherapy (six cycles of isotretinoin and five concomitant cycles of ch14.18 in combination with alternating GM-CSF and interleukin-2). Event-free survival and overall survival were compared between the immunotherapy group and the standard-therapy group, on an intention-to-treat basis.
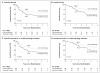
Results: A total of 226 eligible patients were randomly assigned to a treatment group. In the immunotherapy group, a total of 52% of patients had pain of grade 3, 4, or 5, and 23% and 25% of patients had capillary leak syndrome and hypersensitivity reactions, respectively. With 61% of the number of expected events observed, the study met the criteria for early stopping owing to efficacy. The median duration of follow-up was 2.1 years. Immunotherapy was superior to standard therapy with regard to rates of event-free survival (66±5% vs. 46±5% at 2 years, P=0.01) and overall survival (86±4% vs. 75±5% at 2 years, P=0.02 without adjustment for interim analyses).
Conclusions: Immunotherapy with ch14.18, GM-CSF, and interleukin-2 was associated with a significantly improved outcome as compared with standard therapy in patients with high-risk neuroblastoma. (Funded by the National Institutes of Health and the Food and Drug Administration; ClinicalTrials.gov number, NCT00026312.)
Figures
Comment in
-
Antibody therapy and neuroblastoma.N Engl J Med. 2011 Jan 20;364(3):289; author reply 289-90. doi: 10.1056/NEJMc1012160. N Engl J Med. 2011. PMID: 21247330 No abstract available.
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER★Stat mortality database: total US. Bethesda, MD: National Cancer Institute; 1969–2006.
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–20. - PubMed
-
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–73. - PubMed
-
- Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44:5914–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
