Central and peripheral sites of action for CB₂ receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats
- PMID: 20880025
- PMCID: PMC3031063
- DOI: 10.1111/j.1476-5381.2010.01046.x
Central and peripheral sites of action for CB₂ receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats
Abstract
Background and purpose: Cannabinoid CB₂ receptor activation by selective agonists has been shown to produce analgesic effects in preclinical models of inflammatory and neuropathic pain. However, mechanisms underlying CB₂-mediated analgesic effects remain largely unknown. The present study was conducted to elucidate the CB₂ receptor expression in 'pain relevant' tissues and the potential sites of action of CB₂ agonism in rats.
Experimental approach: Expression of cannabinoid receptor mRNA was evaluated by quantitative RT-PCR in dorsal root ganglia (DRGs), spinal cords, paws and several brain regions of sham, chronic inflammatory pain (CFA) and neuropathic pain (spinal nerve ligation, SNL) rats. The sites of CB₂ mediated antinociception were evaluated in vivo following intra-DRG, intrathecal (i.t.) or intraplantar (i.paw) administration of potent CB₂-selective agonists A-836339 and AM1241.
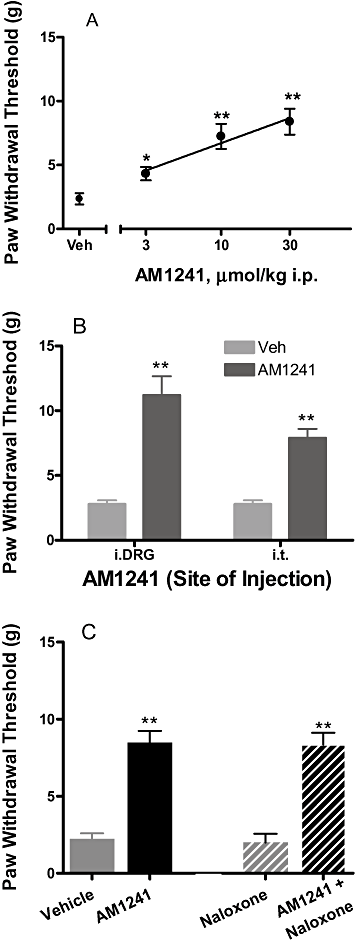
Key results: CB₂ receptor gene expression was significantly up-regulated in DRGs (SNL and CFA), spinal cords (SNL) or paws (CFA) ipsilateral to injury under inflammatory and neuropathic pain conditions. Systemic A-836339 and AM1241 produced dose-dependent efficacy in both inflammatory and neuropathic pain models. Local administration of CB₂ agonists also produced significant analgesic effects in SNL (intra-DRG and i.t.) and CFA (intra-DRG) pain models. In contrast to A-836339, i.paw administration of AM-1241 dose-relatedly reversed the CFA-induced thermal hyperalgesia, suggesting that different mechanisms may be contributing to its in vivo properties.
Conclusions and implications: These results demonstrate that both DRG and spinal cord are important sites contributing to CB₂ receptor-mediated analgesia and that the changes in CB₂ receptor expression play a crucial role for the sites of action in regulating pain perception.
© 2010 The Authors. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures


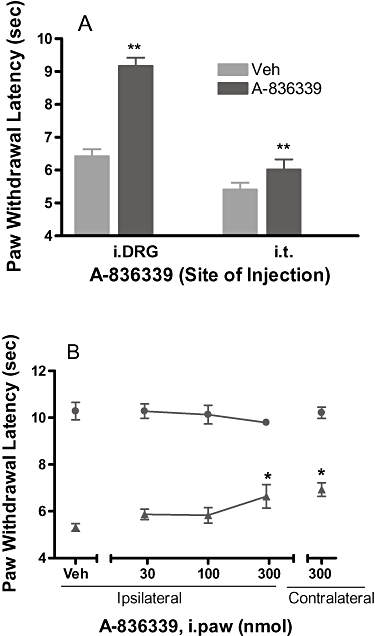

 , veh). (B) Effects of A-836339 on mechanical allodynia in the SNL model of neuropathic pain following iDRG and i.t. administration (100 nmol·rat−1). Data represent mean ± SEM (n = 8). **P < 0.01 as compared with vehicle-treated animals. (C) Lack of naloxone blockade of A-836339 (30 µmol·kg−1 i.p.) reversal of mechanical allodynia. Data represent mean ± SEM (n = 6). **P < 0.01 as compared with vehicle-treated animals. Responses of only the ipsilateral paws of the treated animals were shown. Responses of the respective contralateral paws of all treatment groups are similar to that of the vehicle treated contralateral paws (not shown).
, veh). (B) Effects of A-836339 on mechanical allodynia in the SNL model of neuropathic pain following iDRG and i.t. administration (100 nmol·rat−1). Data represent mean ± SEM (n = 8). **P < 0.01 as compared with vehicle-treated animals. (C) Lack of naloxone blockade of A-836339 (30 µmol·kg−1 i.p.) reversal of mechanical allodynia. Data represent mean ± SEM (n = 6). **P < 0.01 as compared with vehicle-treated animals. Responses of only the ipsilateral paws of the treated animals were shown. Responses of the respective contralateral paws of all treatment groups are similar to that of the vehicle treated contralateral paws (not shown).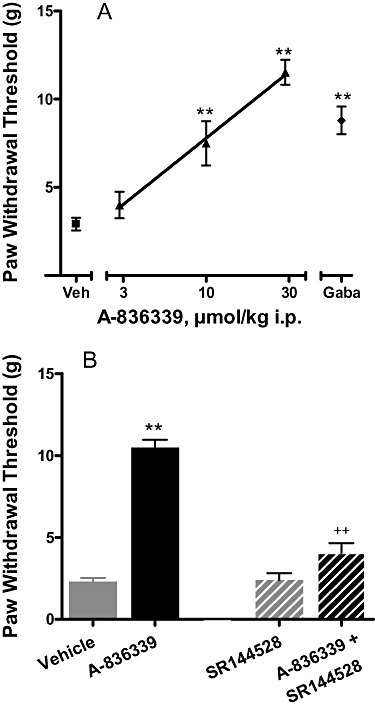
 , veh). (B) Antagonism of the effect of A-836339 (30 µmol·kg−1, i.p.) by SR144528 (10 µmol·kg−1, i.p.). Data represent mean ± SEM (n = 6). **P < 0.01 as compared with vehicle-treated animals, ++P < 0.01 as compared with A-836339 alone. Responses of only the ipsilateral paws of the treated animals were shown. Responses of the respective contralateral paws of all treatment groups are similar to that of the vehicle treated contralateral paws (not shown).
, veh). (B) Antagonism of the effect of A-836339 (30 µmol·kg−1, i.p.) by SR144528 (10 µmol·kg−1, i.p.). Data represent mean ± SEM (n = 6). **P < 0.01 as compared with vehicle-treated animals, ++P < 0.01 as compared with A-836339 alone. Responses of only the ipsilateral paws of the treated animals were shown. Responses of the respective contralateral paws of all treatment groups are similar to that of the vehicle treated contralateral paws (not shown).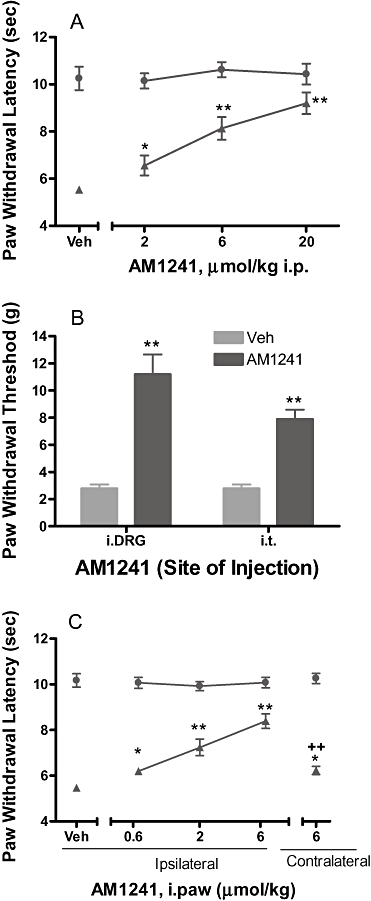
References
-
- Barth F, Casellas P, Congy C, Martinez S, Rinaldi M, Anne-Archard G. Preparation of N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) 4-methylpyrazole-3-carboxamide as a cannabinoid receptor antagonist. European Patent Appl. 1995 EP 656354.
-
- Barth F, Casellas P, Millan J, Oustric D, Rinaldi M, Sarran M. 3-Pyrazolecarboxamide derivatives having cannabinoid receptor. PCT Patent Appl. 1997 WO 9721682.
-
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

