Impact of surface chemistry
- PMID: 20880833
- PMCID: PMC3024684
- DOI: 10.1073/pnas.1006669107
Impact of surface chemistry
Abstract
The applications of molecular surface chemistry in heterogeneous catalyst technology, semiconductor-based technology, medical technology, anticorrosion and lubricant technology, and nanotechnology are highlighted in this perspective. The evolution of surface chemistry at the molecular level is reviewed, and the key roles of surface instrumentation developments for in situ studies of the gas-solid, liquid-solid, and solid-solid interfaces under reaction conditions are emphasized.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

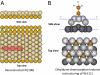

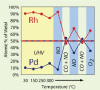


References
-
- Somorjai GA, Li Y. Introduction to Surface Chemistry and Catalysis. 2nd Ed. Hoboken, NJ: Wiley; 2010.
-
- Ertl G. Reactions at Solid Surfaces. Hoboken, NJ: Wiley; 2009.
-
- Adamson AW, Gast AP. Physical Chemistry of Surfaces. 6th Ed. New York: Wiley; 1997.
-
- Hiemenz PC, Rajagopalan R. Principles of Colloid and Surface Chemistry. 3rd Ed. New York: Marcel Dekker; 1997.
-
- Woodruff DP, Delchar TA. Modern Techniques of Surface Science. 2nd Ed. New York: Cambridge Univ Press; 1994.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

