An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals
- PMID: 20880839
- PMCID: PMC2992283
- DOI: 10.1074/jbc.M110.162073
An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals
Abstract
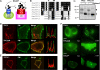
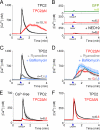
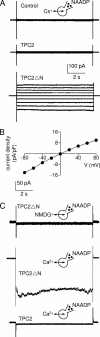
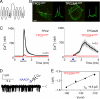
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a ubiquitous messenger proposed to stimulate Ca(2+) release from acidic organelles via two-pore channels (TPCs). It has been difficult to resolve this trigger event from its amplification via endoplasmic reticulum Ca(2+) stores, fuelling speculation that archetypal intracellular Ca(2+) channels are the primary targets of NAADP. Here, we redirect TPC2 from lysosomes to the plasma membrane and show that NAADP evokes Ca(2+) influx independent of ryanodine receptors and that it activates a Ca(2+)-permeable channel whose conductance is reduced by mutation of a residue within a putative pore. We therefore uncouple TPC2 from amplification pathways and prove that it is a pore-forming subunit of an NAADP-gated Ca(2+) channel.
Figures

References
-
- Guse A. H., Lee H. C. (2008) Sci. Signal. 1, re10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

