Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells
- PMID: 20881000
- PMCID: PMC2994281
- DOI: 10.1093/carcin/bgq197
Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells
Abstract
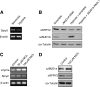
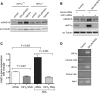
Epigenetic silencing of tumor suppressor genes commonly occurs in human cancers via increasing DNA methylation and repressive histone modifications at gene promoters. However, little is known about how pathogenic environmental factors contribute to cancer development by affecting epigenetic regulatory mechanisms. Previously, we reported that both hypoxia and nickel (an environmental carcinogen) increased global histone H3 lysine 9 methylation in cells through inhibiting a novel class of iron- and α-ketoglutarate-dependent histone demethylases. Here, we investigated whether inhibition of histone demethylase JMJD1A by hypoxia and nickel could lead to repression/silencing of JMJD1A-targeted gene(s). By using Affymetrix GeneChip and ChIP-on-chip technologies, we identified Spry2 gene, a key regulator of receptor tyrosine kinase/extracellular signal-regulated kinase (ERK) signaling, as one of the JMJD1A-targeted genes in human bronchial epithelial BEAS-2B cells. Both hypoxia and nickel exposure increased the level of H3K9me2 at the Spry2 promoter by inhibiting JMJD1A, which probably led to a decreased expression of Spry2 in BEAS-2B cells. Repression of Spry2 potentiated the nickel-induced ERK phosphorylation, and forced expression of Spry2 in BEAS-2B cells decreased the nickel-induced ERK phosphorylation and significantly suppressed nickel-induced anchorage-independent growth. Taken together, our results suggest that histone demethylases could be targets of environmental carcinogens and their inhibition may lead to altered gene expression and eventually carcinogenesis.
Figures





Similar articles
-
Histone demethylase JMJD1A promotes urinary bladder cancer progression by enhancing glycolysis through coactivation of hypoxia inducible factor 1α.Oncogene. 2017 Jul 6;36(27):3868-3877. doi: 10.1038/onc.2017.13. Epub 2017 Mar 6. Oncogene. 2017. PMID: 28263974
-
Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth.Mol Cell Biol. 2010 Jan;30(1):344-53. doi: 10.1128/MCB.00444-09. Mol Cell Biol. 2010. PMID: 19858293 Free PMC article.
-
The expression of histone demethylase JMJD1A in renal cell carcinoma.Neoplasma. 2011;58(2):153-7. doi: 10.4149/neo_2011_02_153. Neoplasma. 2011. PMID: 21275466
-
Pathophysiological response to hypoxia - from the molecular mechanisms of malady to drug discovery: epigenetic regulation of the hypoxic response via hypoxia-inducible factor and histone modifying enzymes.J Pharmacol Sci. 2011;115(4):453-8. doi: 10.1254/jphs.10r19fm. Epub 2011 Mar 16. J Pharmacol Sci. 2011. PMID: 21422728 Review.
-
Nickel carcinogenesis: epigenetics and hypoxia signaling.Mutat Res. 2005 Dec 30;592(1-2):79-88. doi: 10.1016/j.mrfmmm.2005.06.008. Epub 2005 Jul 11. Mutat Res. 2005. PMID: 16009382 Review.
Cited by
-
Histone lysine-specific methyltransferases and demethylases in carcinogenesis: new targets for cancer therapy and prevention.Curr Cancer Drug Targets. 2013 Jun;13(5):558-79. doi: 10.2174/1568009611313050007. Curr Cancer Drug Targets. 2013. PMID: 23713993 Free PMC article. Review.
-
Nickel induces transcriptional down-regulation of DNA repair pathways in tumorigenic and non-tumorigenic lung cells.Carcinogenesis. 2017 Jun 1;38(6):627-637. doi: 10.1093/carcin/bgx038. Carcinogenesis. 2017. PMID: 28472268 Free PMC article.
-
Epigenetic Regulation in Exposome-Induced Tumorigenesis: Emerging Roles of ncRNAs.Biomolecules. 2022 Mar 28;12(4):513. doi: 10.3390/biom12040513. Biomolecules. 2022. PMID: 35454102 Free PMC article. Review.
-
Epigenetic mechanisms in metal carcinogenesis.Toxicol Rep. 2022 Apr 4;9:778-787. doi: 10.1016/j.toxrep.2022.03.037. eCollection 2022. Toxicol Rep. 2022. PMID: 36561948 Free PMC article. Review.
-
Effects of Nickel Bioaccumulation on Hematological, Biochemical, Immune Responses, Neuroinflammatory, Oxidative Stress Parameters, and Neurotoxicity in Rats.Biol Trace Elem Res. 2025 Sep;203(9):4707-4727. doi: 10.1007/s12011-025-04528-x. Epub 2025 Feb 1. Biol Trace Elem Res. 2025. PMID: 39891830
References
-
- Gluckman PD, et al. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009;5:401–408. - PubMed
-
- Herceg Z. Epigenetics and cancer: towards an evaluation of the impact of environmental and dietary factors. Mutagenesis. 2007;22:91–103. - PubMed
-
- Chen H, et al. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006;66:9009–9016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

