Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade
- PMID: 20881034
- PMCID: PMC3006314
- DOI: 10.1152/ajprenal.00153.2010
Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade
Abstract
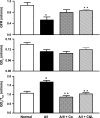
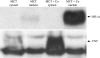
The 5/6(th) nephrectomy or ablation/infarction (A/I) preparation has been used as a classic model of chronic kidney disease (CKD). We observed increased kidney oxygen consumption (Q(O2)) and altered renal hemodynamics in the A/I kidney that were normalized after combined angiotensin II (ANG II) blockade. Studies suggest hypoxia inducible factor as a protective influence in A/I. We induced hypoxia-inducible factor (HIF) and HIF target proteins by two different methods, cobalt chloride (CoCl(2)) and dimethyloxalyglycine (DMOG), for the first week after creation of A/I and compared the metabolic and renal hemodynamic outcomes to combined ANG II blockade. We also examined the HIF target proteins expressed by using Western blots and real-time PCR. Treatment with DMOG, CoCl(2), and ANG II blockade normalized kidney oxygen consumption factored by Na reabsorption and increased both renal blood flow and glomerular filtration rate. At 1 wk, CoCl(2) and DMOG increased kidney expression of HIF by Western blot. In the untreated A/I kidney, VEGF, heme oxygenase-1, and GLUT1 were all modestly increased. Both ANG II blockade and CoCl(2) therapy increased VEGF and GLUT1 but the cobalt markedly so. ANG II blockade decreased heme oxygenase-1 expression while CoCl(2) increased it. By real-time PCR, erythropoietin and GLUT1 were only increased by CoCl(2) therapy. Cell proliferation was modestly increased by ANG II blockade but markedly after cobalt therapy. Metabolic and hemodynamic abnormalities were corrected equally by ANG II blockade and HIF therapies. However, the molecular patterns differed significantly between ANG II blockade and cobalt therapy. HIF induction may prove to be protective in this model of CKD.
Figures

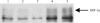





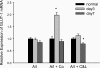


Similar articles
-
Increased activation of the hypoxia-inducible factor pathway in varicose veins.J Vasc Surg. 2012 May;55(5):1427-39. doi: 10.1016/j.jvs.2011.10.111. Epub 2012 Jan 24. J Vasc Surg. 2012. PMID: 22277691
-
Hypoxia and metabolic phenotypes during breast carcinogenesis: expression of HIF-1alpha, GLUT1, and CAIX.Virchows Arch. 2010 Jul;457(1):53-61. doi: 10.1007/s00428-010-0938-0. Epub 2010 Jun 5. Virchows Arch. 2010. PMID: 20526721
-
Hypoxia-inducible factor-1α activation improves renal oxygenation and mitochondrial function in early chronic kidney disease.Am J Physiol Renal Physiol. 2017 Aug 1;313(2):F282-F290. doi: 10.1152/ajprenal.00579.2016. Epub 2017 Mar 22. Am J Physiol Renal Physiol. 2017. PMID: 28331062 Free PMC article.
-
Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis.Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1337-45. doi: 10.1089/ars.2005.7.1337. Antioxid Redox Signal. 2005. PMID: 16115039 Review.
-
Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics.Am J Kidney Dis. 2021 Feb;77(2):280-286. doi: 10.1053/j.ajkd.2020.04.016. Epub 2020 Jul 23. Am J Kidney Dis. 2021. PMID: 32711072 Review.
Cited by
-
Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension.Clin Exp Pharmacol Physiol. 2013 Feb;40(2):123-37. doi: 10.1111/1440-1681.12034. Clin Exp Pharmacol Physiol. 2013. PMID: 23181475 Free PMC article. Review.
-
The Role of Hypoxia-Inducible Factor/Prolyl Hydroxylation Pathway in Deoxycorticosterone Acetate/Salt Hypertension in the Rat.J Hypertens (Los Angel). 2014 Dec;3(6):184. doi: 10.4172/2167-1095.1000184. J Hypertens (Los Angel). 2014. PMID: 26185735 Free PMC article.
-
FIH-1-Mint3 axis does not control HIF-1 transcriptional activity in nucleus pulposus cells.J Biol Chem. 2014 Jul 25;289(30):20594-605. doi: 10.1074/jbc.M114.565101. J Biol Chem. 2014. PMID: 24867948 Free PMC article.
-
Regeneration in the nervous system with erythropoietin.Front Biosci (Landmark Ed). 2016 Jan;21(3):561-596. doi: 10.2741/4408. Front Biosci (Landmark Ed). 2016. PMID: 26549969 Free PMC article.
-
Targeting HIF-1α to Prevent Renal Ischemia-Reperfusion Injury: Does It Work?Int J Cell Biol. 2018 Nov 25;2018:9852791. doi: 10.1155/2018/9852791. eCollection 2018. Int J Cell Biol. 2018. PMID: 30595695 Free PMC article. Review.
References
-
- Aizawa T, Ishizaka N, Taguchi J, Nagai R, Mori I, Tang SS, Ingelfinger JR, Ohno M. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension 35: 800– 806, 2000 - PubMed
-
- Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570– H578, 2008 - PubMed
-
- Badr GA, Zhang JZ, Tang J, Kern TS, Ismail-Beigi F. Glut1 and glut3 expression, but not capillary density, is increased by cobalt chloride in rat cerebrum and retina. Brain Res Mol Brain Res 64: 24– 33, 1999 - PubMed
-
- Beck I, Weinmann R, Caro J. Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood 82: 704– 711, 1993 - PubMed
-
- Bernhardt WM, Gottmann U, Doyon F, Buchholz B, Campean V, Schodel J, Reisenbuechler A, Klaus S, Arend M, Flippin L, Willam C, Wiesener MS, Yard B, Warnecke C, Eckardt KU. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc Natl Acad Sci USA 106: 21276– 21281, 2009 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

