Intrinsic cytoskeleton-dependent clustering of influenza virus M2 protein with hemagglutinin assessed by FLIM-FRET
- PMID: 20881046
- PMCID: PMC2976392
- DOI: 10.1128/JVI.01322-10
Intrinsic cytoskeleton-dependent clustering of influenza virus M2 protein with hemagglutinin assessed by FLIM-FRET
Abstract
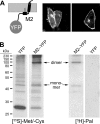
The hemagglutinin (HA) of influenza virus organizes the virus bud zone, a domain of the plasma membrane enriched in raft lipids. Using fluorescence lifetime imaging microscopy-fluorescence resonance energy transfer (FLIM-FRET), a technique that detects close colocalization of fluorescent proteins in transfected cells, we show that the viral proton channel M2 clusters with HA but not with a marker for inner leaflet rafts. The FRET signal between M2 and HA depends on the raft-targeting signals in HA and on an intact actin cytoskeleton. We conclude that M2 contains an intrinsic signal that targets the protein to the viral bud zone, which is organized by raft-associated HA and by cortical actin.
Figures

References
-
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821-1825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

