High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats
- PMID: 20881195
- PMCID: PMC3007662
- DOI: 10.1152/jn.00663.2010
High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats
Abstract
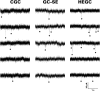
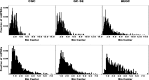
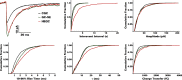
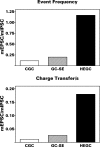
After experimental status epilepticus, many dentate granule cells born into the postseizure environment migrate aberrantly into the dentate hilus. Hilar ectopic granule cells (HEGCs) have also been found in persons with epilepsy. These cells exhibit a high rate of spontaneous activity, which may enhance seizure propagation. Electron microscopic studies indicated that HEGCs receive more recurrent mossy fiber innervation than normotopic granule cells in the same animals but receive much less inhibitory innervation. This study used hippocampal slices prepared from rats that had experienced pilocarpine-induced status epilepticus to test the hypothesis that an imbalance of synaptic excitation and inhibition contributes to the hyperexcitability of HEGCs. Mossy fiber stimulation evoked a much smaller GABA(A) receptor-mediated inhibitory postsynaptic currents (IPSC) in HEGCs than in normotopic granule cells from either control rats or rats that had experienced status epilepticus. However, recurrent mossy fiber-evoked excitatory postsynaptic currents (EPSCs) of similar size were recorded from HEGCs and normotopic granule cells in status epilepticus-experienced rats. HEGCs exhibited the highest frequency of miniature excitatory postsynaptic currents (mEPSCs) and the lowest frequency of miniature inhibitory postsynaptic currents (mIPSCs) of any granule cell group. On average, both mEPSCs and mIPSCs were of higher amplitude, transferred more charge per event, and exhibited slower kinetics in HEGCs than in granule cells from control rats. Charge transfer per unit time in HEGCs was greater for mEPSCs and much less for mIPSCs than in the normotopic granule cell groups. A high ratio of excitatory to inhibitory synaptic function probably accounts, in part, for the hyperexcitability of HEGCs.
Figures




References
-
- Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to spontaneous recurrent seizures in the rat lithium pilocarpine model of temporal lobe epilepsy. Hippocampus 11: 452–468, 2001 - PubMed
-
- Bonde S, Ekdahl CT, Lindvall O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci 23: 965–974, 2006 - PubMed
-
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 4: 1166–1172, 1998 - PubMed
-
- Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol 77: 2685–2696, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

