Linear hypergeneralization of learned dynamics across movement speeds reveals anisotropic, gain-encoding primitives for motor adaptation
- PMID: 20881197
- PMCID: PMC3023379
- DOI: 10.1152/jn.00884.2009
Linear hypergeneralization of learned dynamics across movement speeds reveals anisotropic, gain-encoding primitives for motor adaptation
Abstract
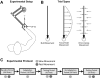
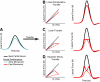
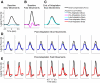
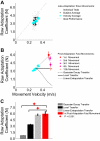
The ability to generalize learned motor actions to new contexts is a key feature of the motor system. For example, the ability to ride a bicycle or swing a racket is often first developed at lower speeds and later applied to faster velocities. A number of previous studies have examined the generalization of motor adaptation across movement directions and found that the learned adaptation decays in a pattern consistent with the existence of motor primitives that display narrow Gaussian tuning. However, few studies have examined the generalization of motor adaptation across movement speeds. Following adaptation to linear velocity-dependent dynamics during point-to-point reaching arm movements at one speed, we tested the ability of subjects to transfer this adaptation to short-duration higher-speed movements aimed at the same target. We found near-perfect linear extrapolation of the trained adaptation with respect to both the magnitude and the time course of the velocity profiles associated with the high-speed movements: a 69% increase in movement speed corresponded to a 74% extrapolation of the trained adaptation. The close match between the increase in movement speed and the corresponding increase in adaptation beyond what was trained indicates linear hypergeneralization. Computational modeling shows that this pattern of linear hypergeneralization across movement speeds is not compatible with previous models of adaptation in which motor primitives display isotropic Gaussian tuning of motor output around their preferred velocities. Instead, we show that this generalization pattern indicates that the primitives involved in the adaptation to viscous dynamics display anisotropic tuning in velocity space and encode the gain between motor output and motion state rather than motor output itself.
Figures


Similar articles
-
Error generalization as a function of velocity and duration: human reaching movements.Exp Brain Res. 2008 Mar;186(1):23-37. doi: 10.1007/s00221-007-1202-y. Epub 2007 Nov 20. Exp Brain Res. 2008. PMID: 18030456
-
On the encoding capacity of human motor adaptation.J Neurophysiol. 2021 Jul 1;126(1):123-139. doi: 10.1152/jn.00593.2020. Epub 2021 Jun 2. J Neurophysiol. 2021. PMID: 34077281
-
Primitives for motor adaptation reflect correlated neural tuning to position and velocity.Neuron. 2009 Nov 25;64(4):575-89. doi: 10.1016/j.neuron.2009.10.001. Neuron. 2009. PMID: 19945398
-
Sensorimotor aspects of high-speed artificial gravity: III. Sensorimotor adaptation.J Vestib Res. 2002-2003;12(5-6):291-9. J Vestib Res. 2002. PMID: 14501105 Review.
-
Primitives, premotor drives, and pattern generation: a combined computational and neuroethological perspective.Prog Brain Res. 2007;165:323-46. doi: 10.1016/S0079-6123(06)65020-6. Prog Brain Res. 2007. PMID: 17925255 Review.
Cited by
-
The training schedule affects the stability, not the magnitude, of the interlimb transfer of learned dynamics.J Neurophysiol. 2013 Aug;110(4):984-98. doi: 10.1152/jn.01072.2012. Epub 2013 May 29. J Neurophysiol. 2013. PMID: 23719204 Free PMC article.
-
Separate contributions of kinematic and kinetic errors to trajectory and grip force adaptation when transporting novel hand-held loads.J Neurosci. 2013 Jan 30;33(5):2229-36. doi: 10.1523/JNEUROSCI.3772-12.2013. J Neurosci. 2013. PMID: 23365258 Free PMC article. Clinical Trial.
-
Paradoxical benefits of dual-task contexts for visuomotor memory.Psychol Sci. 2015 Feb;26(2):148-58. doi: 10.1177/0956797614557868. Epub 2014 Dec 10. Psychol Sci. 2015. PMID: 25501806 Free PMC article.
-
Generalizing movement patterns following shoulder fixation.J Neurophysiol. 2020 Mar 1;123(3):1193-1205. doi: 10.1152/jn.00696.2019. Epub 2020 Feb 26. J Neurophysiol. 2020. PMID: 32101490 Free PMC article.
-
The cerebellum acts as the analog to the medial temporal lobe for sensorimotor memory.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2411459121. doi: 10.1073/pnas.2411459121. Epub 2024 Oct 7. Proc Natl Acad Sci U S A. 2024. PMID: 39374383 Free PMC article.
References
-
- Bedford FL. Perceptual and cognitive spatial learning. J Exp Psychol Hum Percept Perform 19: 517–530, 1993 - PubMed
-
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635, 1985 - PubMed
-
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734, 1985 - PubMed
-
- Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol 96: 3130–3146, 2006 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

