Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer
- PMID: 20881251
- PMCID: PMC2954725
- DOI: 10.1210/en.2010-0436
Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer
Abstract
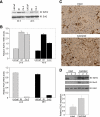
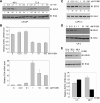
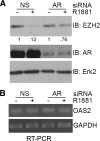
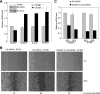
Androgens and the androgen receptor are important for both normal prostate development and progression of prostate cancer (PCa). However, the underlying mechanisms are not fully understood. The Polycomb protein enhancer of zeste homolog 2 (EZH2) functions as an epigenetic gene silencer and plays a role in oncogenesis by promoting cell proliferation and invasion. EZH2 has been implicated in human PCa progression, because its expression is often elevated in hormone-refractory PCa. Here, we demonstrated that expression of EZH2 is lower in androgen-sensitive LNCaP PCa cells compared with Rf and C4-2 cells, two androgen-refractory sublines that are derived from LNCaP cells. Androgen ablation by castration increased the level of EZH2 proteins in LNCaP xenografts in mice. In contrast, treatment of LNCaP cells in culture with the synthetic androgen methyltrieolone (R1881) at doses of 1 nm or higher suppressed EZH2 expression. Moreover, our data suggest that androgen repression of EZH2 requires a functional androgen receptor and this effect is mediated through the retinoblastoma protein and its related protein p130. We further showed that androgen treatment not only increases expression of EZH2 target genes DAB2IP and E-cadherin but also affects LNCaP cell migration. Our results reveal that androgens function as an epigenetic regulator in prostatic cells by repression of EZH2 expression through the retinoblastoma protein and p130-dependent pathways. Our findings also suggest that blockade of EZH2 derepression during androgen deprivation therapy may represent an effective tactic for the treatment of androgen-refractory PCa.
Figures


Similar articles
-
MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta.Mol Cancer. 2010 May 17;9:108. doi: 10.1186/1476-4598-9-108. Mol Cancer. 2010. PMID: 20478051 Free PMC article.
-
Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer.J Biol Chem. 2005 Jun 10;280(23):22437-44. doi: 10.1074/jbc.M501379200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15817459
-
Developmental and androgenic regulation of chromatin regulators EZH2 and ANCCA/ATAD2 in the prostate Via MLL histone methylase complex.Prostate. 2013 Apr;73(5):455-66. doi: 10.1002/pros.22587. Epub 2012 Oct 4. Prostate. 2013. PMID: 23038103
-
Importance of Ezh2 polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements.J Cell Physiol. 2008 Feb;214(2):295-300. doi: 10.1002/jcp.21241. J Cell Physiol. 2008. PMID: 17786943 Review.
-
Androgen action in the prostate gland.Minerva Urol Nefrol. 2012 Mar;64(1):35-49. Minerva Urol Nefrol. 2012. PMID: 22402316 Review.
Cited by
-
Androgen Receptor Tumor Suppressor Function Is Mediated by Recruitment of Retinoblastoma Protein.Cell Rep. 2016 Oct 18;17(4):966-976. doi: 10.1016/j.celrep.2016.09.064. Cell Rep. 2016. PMID: 27760327 Free PMC article.
-
Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3.Oncotarget. 2016 Jun 21;7(25):37868-37881. doi: 10.18632/oncotarget.9350. Oncotarget. 2016. PMID: 27191265 Free PMC article.
-
Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers.Nat Commun. 2018 Oct 4;9(1):4080. doi: 10.1038/s41467-018-06177-2. Nat Commun. 2018. PMID: 30287808 Free PMC article.
-
The AR dependent cell cycle: mechanisms and cancer relevance.Mol Cell Endocrinol. 2012 Apr 16;352(1-2):34-45. doi: 10.1016/j.mce.2011.06.033. Epub 2011 Jul 12. Mol Cell Endocrinol. 2012. PMID: 21782001 Free PMC article. Review.
-
Scaffold attachment factor B1: an intrinsic inhibitor of androgen receptor downregulated in prostate cancer.Asian J Androl. 2013 Nov;15(6):703-4. doi: 10.1038/aja.2013.116. Epub 2013 Sep 30. Asian J Androl. 2013. PMID: 24077602 Free PMC article.
References
-
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y 1987 The endocrinology and developmental biology of the prostate. Endocr Rev 8:338–362 - PubMed
-
- Kyprianou N, Isaacs JT 1988 Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 122:552–562 - PubMed
-
- Huang H, Tindall DJ 2002 The role of the androgen receptor in prostate cancer. Crit Rev Eukar Gene 12:193–207 - PubMed
-
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 - PubMed
-
- Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM 2007 Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 448:595–599 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

