Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism
- PMID: 20881259
- PMCID: PMC3038485
- DOI: 10.1210/jc.2009-2682
Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism
Abstract
Context: The osteoanabolic properties of PTH may be due to increases in the number and maturity of circulating osteogenic cells. Hypoparathyroidism is a useful clinical model because this hypothesis can be tested by administering PTH.
Objective: The objective of the study was to characterize circulating osteogenic cells in hypoparathyroid subjects during 12 months of PTH (1-84) administration.
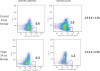
Design: Osteogenic cells were characterized using flow cytometry and antibodies against osteocalcin, an osteoblast-specific protein product, and stem cell markers CD34 and CD146. Changes in bone formation from biochemical markers and quadruple-labeled transiliac crest bone biopsies (0 and 3 month time points) were correlated with measurements of circulating osteogenic cells.
Setting: The study was conducted at a clinical research center.
Patients: Nineteen control and 19 hypoparathyroid patients were included in the study.
Intervention: Intervention included the administration of PTH (1-84).
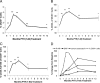
Results: Osteocalcin-positive cells were lower in hypoparathyroid subjects than controls (0.7 ± 0.1 vs. 2.0 ± 0.1%; P < 0.0001), with greater coexpression of the early cell markers CD34 and CD146 among the osteocalcin-positive cells in the hypoparathyroid subjects (11.0 ± 1.0 vs. 5.6 ± 0.7%; P < 0.001). With PTH (1-84) administration, the number of osteogenic cells increased 3-fold (P < 0.0001), whereas the coexpression of the early cell markers CD34 and CD146 decreased. Increases in osteogenic cells correlated with circulating and histomorphometric indices of osteoblast function: N-terminal propeptide of type I procollagen (R(2) = 0.4, P ≤ 0.001), bone-specific alkaline phosphatase (R(2) = 0.3, P < 0.001), osteocalcin (R(2) = 0.4, P < 0.001), mineralized perimeter (R(2) = 0.5, P < 0.001), mineral apposition rate (R(2) = 0.4, P = 0.003), and bone formation rate (R(2) = 0.5, P < 0.001).
Conclusions: It is likely that PTH stimulates bone formation by stimulating osteoblast development and maturation. Correlations between circulating osteogenic cells and histomorphometric indices of bone formation establish that osteoblast activity is being identified by this methodology.
Figures



References
-
- Suda T, Takahashi N 1996 Cells of bone: osteoclast generation. In: Bilezikian J, Rodan GA, eds. Principles of bone biology. New York: Academic Press; 87–102
-
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 - PubMed
-
- Aubin J 2001 Cellular actions of parathyroid hormone on osteoblast and osteoclast differentiation. In: Bilezikian J, Levine MA, eds. The parathyroids. San Diego: Academic Press; 199–211
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

