A phase II first-line study of gemcitabine, carboplatin, and bevacizumab in advanced stage nonsquamous non-small cell lung cancer
- PMID: 20881641
- PMCID: PMC4241413
- DOI: 10.1097/JTO.0b013e3181f1d23c
A phase II first-line study of gemcitabine, carboplatin, and bevacizumab in advanced stage nonsquamous non-small cell lung cancer
Abstract
Background: Bevacizumab improves responses and progression-free survival when added to first-line paclitaxel/carboplatin or cisplatin/gemcitabine for patients with advanced nonsquamous non-small cell lung cancer. This study was designed to evaluate toxicities and efficacy of gemcitabine/carboplatin/bevacizumab.
Methods: Patients with untreated advanced nonsquamous non-small cell lung cancer, with no evidence of brain metastases and not on anticoagulation were eligible. Patients received gemcitabine 1000 mg/m on days 1 and 8; carboplatin area under the curve 5 day 1; and bevacizumab 15 mg/kg day 1 every 3 weeks for up to six cycles. Bevacizumab was then continued every 3 weeks until disease progression or unacceptable toxicity.
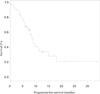
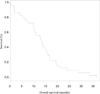
Results: From July 2006 to December 2008, 48 patients were enrolled: 23 (48%) men, 25 (52%) women, and 19 (40%) never smokers. One patient never received therapy and is not included in the analysis. Median cycle number was 8 (1-42) with 37 patients (78.7%) completing ≥4 cycles of three drugs. Dose reductions occurred in 34 (72.3%) patients. Grade 3/4 toxicities included neutropenia (47%/15%), thrombocytopenia (11%/15%), anemia (6%/0%), dyspnea (6%/2%), bacterial pneumonia (4%/0%), and hypertension (4%/2%). No neutropenic fevers occurred. One patient died of hemoptysis. Grade 3 bleeding occurred in three other patients. There were seven (14.9%) partial responses. Median time to first event (progression/death/toxicity requiring discontinuation) was 6.4 months (95% confidence interval: 4.8-7.9 months). The median overall survival (OS) was 12.8 months (95% confidence interval: 10.0-16.5). The OS is 57% at 1 year and 10% at 2 years.
Conclusions: Although perhaps skewed by a high proportion of nonsmokers and women, treatment with gemcitabine/carboplatin/bevacizumab has an acceptable toxicity profile with promising median OS despite a low response rate.
Figures
Similar articles
-
Bortezomib plus gemcitabine/carboplatin as first-line treatment of advanced non-small cell lung cancer: a phase II Southwest Oncology Group Study (S0339).J Thorac Oncol. 2009 Jan;4(1):87-92. doi: 10.1097/JTO.0b013e3181915052. J Thorac Oncol. 2009. PMID: 19096312 Free PMC article. Clinical Trial.
-
Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer.J Thorac Oncol. 2009 Dec;4(12):1537-43. doi: 10.1097/JTO.0b013e3181c0a2f4. J Thorac Oncol. 2009. PMID: 19887966 Clinical Trial.
-
A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer.J Thorac Oncol. 2012 Jan;7(1):196-202. doi: 10.1097/JTO.0b013e3182307efe. J Thorac Oncol. 2012. PMID: 21900836 Clinical Trial.
-
Phase II study of first-line sequential chemotherapy with gemcitabine-carboplatin followed by docetaxel in patients with advanced non-small cell lung cancer.Oncology. 2005;68(4-6):382-90. doi: 10.1159/000086979. Epub 2005 Jul 12. Oncology. 2005. PMID: 16020967 Review.
-
Antibodies to vascular endothelial growth factor in non-small cell lung cancer.J Thorac Oncol. 2008 Jun;3(6 Suppl 2):S113-8. doi: 10.1097/JTO.0b013e318174e993. J Thorac Oncol. 2008. PMID: 18520292 Review.
Cited by
-
Antiangiogenic agents in combination with chemotherapy in patients with advanced non-small cell lung cancer.Cancer Invest. 2011 May;29(4):325-37. doi: 10.3109/07357907.2011.554476. Cancer Invest. 2011. PMID: 21469981 Free PMC article. Review.
-
The high incidence of vascular thromboembolic events in patients with metastatic or unresectable urothelial cancer treated with platinum chemotherapy agents.Cancer. 2016 Mar 1;122(5):712-21. doi: 10.1002/cncr.29801. Epub 2015 Nov 30. Cancer. 2016. PMID: 26618338 Free PMC article.
-
Feasibility of cisplatin/pemetrexed with 15 mg/kg bevacizumab for the treatment of patients with advanced non-squamous non-small cell lung cancer.Oncol Lett. 2015 Jun;9(6):2577-2582. doi: 10.3892/ol.2015.3086. Epub 2015 Mar 30. Oncol Lett. 2015. PMID: 26137109 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Rami-Porta R, Chansky K, Goldstraw P. Updated lung cancer staging system. Future Oncol. 2009;5:1545–1553. - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
-
- Manegold C. Bevacizumab for the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2008;8:689–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical