Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer
- PMID: 20881962
- PMCID: PMC3084017
- DOI: 10.1038/nature09387
Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer
Abstract
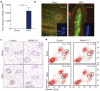
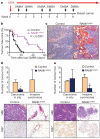
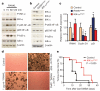
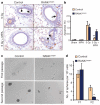
Breast cancer is one of the most common cancers in humans and will on average affect up to one in eight women in their lifetime in the United States and Europe. The Women's Health Initiative and the Million Women Study have shown that hormone replacement therapy is associated with an increased risk of incident and fatal breast cancer. In particular, synthetic progesterone derivatives (progestins) such as medroxyprogesterone acetate (MPA), used in millions of women for hormone replacement therapy and contraceptives, markedly increase the risk of developing breast cancer. Here we show that the in vivo administration of MPA triggers massive induction of the key osteoclast differentiation factor RANKL (receptor activator of NF-κB ligand) in mammary-gland epithelial cells. Genetic inactivation of the RANKL receptor RANK in mammary-gland epithelial cells prevents MPA-induced epithelial proliferation, impairs expansion of the CD49f(hi) stem-cell-enriched population, and sensitizes these cells to DNA-damage-induced cell death. Deletion of RANK from the mammary epithelium results in a markedly decreased incidence and delayed onset of MPA-driven mammary cancer. These data show that the RANKL/RANK system controls the incidence and onset of progestin-driven breast cancer.
Figures
Comment in
-
Tumorigenesis: Joining the RANKs.Nat Rev Cancer. 2010 Nov;10(11):738. doi: 10.1038/nrc2955. Nat Rev Cancer. 2010. PMID: 21080581 No abstract available.
-
Cancer: RANKL inhibition—a new weapon against breast cancer?Nat Rev Endocrinol. 2011 Jan;7(1):2. doi: 10.1038/nrendo.2010.202. Nat Rev Endocrinol. 2011. PMID: 21197701 No abstract available.
-
High hopes for RANKL: will the mouse model live up to its promise?Breast Cancer Res. 2011 Jan 28;13(1):302. doi: 10.1186/bcr2805. Breast Cancer Res. 2011. PMID: 21345281 Free PMC article.
-
A local basis for progesterone action during mammary tumorigenesis - no longer RANK and file.Breast Cancer Res. 2011 Jan 28;13(1):301. doi: 10.1186/bcr2802. Breast Cancer Res. 2011. PMID: 21345282 Free PMC article.
References
-
- Jemal A, et al. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. - PubMed
-
- Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc. 2002;288:321–333. - PubMed
-
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. - PubMed
-
- Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

