Aromatic Nonpolar Nucleosides as Hydrophobic Isosteres of Pyrimidine and Purine Nucleosides
- PMID: 20882116
- PMCID: PMC2946157
- DOI: 10.1021/jo00103a013
Aromatic Nonpolar Nucleosides as Hydrophobic Isosteres of Pyrimidine and Purine Nucleosides
Abstract
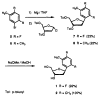
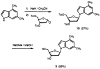
Described are the design, synthesis, and structures of three nonpolar nucleoside isosteres to be used as probes of noncovalent bonding in DNA and as isosteric replacements for the natural nucleosides in designed nucleic acid structures. Reaction of substituted aryl Grignards with 3',5'-bis-O-toluoyl-α-deoxyibofuranosyl chloride and subsequent deprotection with sodium methoxide in methanol afforded the two β-C-nucleoside pyrimidine analogs 1 and 2. The dimethylindolyl nucleoside 3, a purine isostere, was obtained by a nucleophilic displacement on α-chlorodeoxyribofuranose by the sodium salt of 4,6-dimethylindole, followed by deprotection. Regio- and stereochemistry of the products were established with NOE difference spectra and (1)H NMR splitting patterns. Analogs 1 and 2 are nonpolar isosteres of thymidine, and nucleoside 3 is an isostere of 2-aminodeoxyadenosine, the triply-bonded Watson-Crick partner of thymidine. Semiempirical AM1 calculations were carried out to provide bond length information to assess structural similarities between the isosteres and their natural counterparts.
Figures
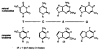

References
-
-
For recent examples, see: Petti M, Shepodd T, Barrans R, Dougherty D. J Am Chem Soc. 1988;110:6825–6840.Cowart M, Sucholeiki I, Bukownik R, Wilcox C. J Am Chem Soc. 1988;110:6204–6210.Smithrud D, Wyman T, Diederich F. J Am Chem Soc. 1991;113:5420–5426.Rotello VM, Viani EA, Deslongchamps G, Murray BA, Rebek J. J Am Chem Soc. 1993;115:797–798.Newcomb LF, Gellman SH. J Am Chem Soc. 1994;116:4993–4994.
-
-
- Klotz IM, Frantzen JS. J Am Chem Soc. 1962;84:3461.
- Cantor CR, Schimmel PR. Biophysical Chemistry Part I: The Conformation of Biological Macromolecules. W. H. Freeman; San Francisco: 1980. pp. 277–279.
-
- Cantor CR, Schimmel PR. Biophysical Chemistry Part III: The Behavior of Biological Macromolecules. W. H. Freeman; San Francisco: 1980. pp. 1117–1133.
-
- Petersheim M, Turner DH. Biochemistry. 1988;22:256–263. - PubMed
-
- Senior M, Jones RA, Breslauer KJ. Biochemistry. 1988;27:3879–3885. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
