Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial
- PMID: 20882268
- PMCID: PMC2995871
- DOI: 10.1007/s00125-010-1922-6
Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial
Erratum in
- Diabetologia. 2011 Aug;54(8):2209
Abstract
Aims/hypothesis: To assess the effect of an angiotensin receptor blocker (ARB) on serum potassium and the effect of a serum potassium change on renal outcomes in patients with type 2 diabetes and nephropathy.
Methods: We performed a post hoc analysis in patients with type 2 diabetes participating in the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study. Renal outcomes were defined as a composite of doubling of serum creatinine or end-stage renal disease.
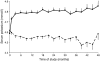
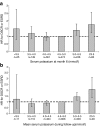
Results: At month 6, 259 (38.4%) and 73 (10.8%) patients in the losartan group and 151 (22.8%) and 34 (5.1%) patients in the placebo group had serum potassium ≥5.0 mmol/l and ≥5.5 mmol/l, (p < 0.001), respectively. Losartan was an independent predictor for serum potassium ≥5.0 mmol/l at month 6 (OR 2.8; 95% CI 2.0-3.9). Serum potassium at month 6 ≥ 5.0 mmol/l was in turn associated with increased risk for renal events (HR 1.22; 95% CI 1.00-1.50), independent of other risk factors. Adjustment of the overall treatment effects for serum potassium augmented losartan's renoprotective effect from 21% (6-34%) to 35% (20-48%), suggesting that the renoprotective effects of losartan are offset by its effect on serum potassium.
Conclusions/interpretation: In this study, we found that treatment with the ARB losartan is associated with a high risk of increased serum potassium levels, which is in turn associated with an increased risk of renal outcomes in patients with diabetes and nephropathy. Whether additional management of high serum potassium would further increase the renal protective properties of losartan is an important clinical question.
Figures
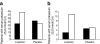
Comment in
-
Diabetes: Treatment with losartan increases risk of adverse renal outcomes.Nat Rev Endocrinol. 2011 Jan;7(1):2. doi: 10.1038/nrendo.2010.203. Nat Rev Endocrinol. 2011. PMID: 21197702 No abstract available.
-
High serum potassium levels after using losartan can reflect more severe renal disease.Diabetologia. 2011 Nov;54(11):2963-4; author reply 2965-7. doi: 10.1007/s00125-011-2220-7. Epub 2011 Jun 18. Diabetologia. 2011. PMID: 21688085 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

