Hydrogels in regenerative medicine
- PMID: 20882499
- PMCID: PMC4494665
- DOI: 10.1002/adma.200802106
Hydrogels in regenerative medicine
Abstract
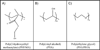
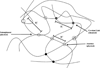
Hydrogels, due to their unique biocompatibility, flexible methods of synthesis, range of constituents, and desirable physical characteristics, have been the material of choice for many applications in regenerative medicine. They can serve as scaffolds that provide structural integrity to tissue constructs, control drug and protein delivery to tissues and cultures, and serve as adhesives or barriers between tissue and material surfaces. In this work, the properties of hydrogels that are important for tissue engineering applications and the inherent material design constraints and challenges are discussed. Recent research involving several different hydrogels polymerized from a variety of synthetic and natural monomers using typical and novel synthetic methods are highlighted. Finally, special attention is given to the microfabrication techniques that are currently resulting in important advances in the field.
Figures






References
-
- Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perezatayde A, Langer R. J Pediatr. Surg. 1988;23:3. - PubMed
-
-
Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997–2006, Department of Health and Human Services (HHS), Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD 2007. The data and analyses reported in this report have been supplied by the United Network for Organ Sharing, VA and Arbor Research Collaborative for Health, MI under contract with HHS. The authors alone are responsible for reporting and interpreting these data.
-
-
- Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Annu. Rev. Biomed. Eng. 2000;2:9. - PubMed
-
- Lee KY, Mooney DJ. Chem. Rev. 2001;101:1869. - PubMed
-
- Malafaya PB, Silva GA, Reis RL. Adv. Drug Delivery Rev. 2007;59:207. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

