Building and remodeling synapses
- PMID: 20882551
- PMCID: PMC3090530
- DOI: 10.1002/hipo.20872
Building and remodeling synapses
Abstract
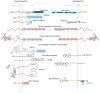
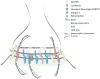
Synaptic junctions are generated by adhesion proteins that bridge the synaptic cleft to firmly anchor pre- and postsynaptic membranes. Several cell adhesion molecule (CAM) families localize to synapses, but it is not yet completely understood how each synaptic CAM family contributes to synapse formation and/or structure, and whether or how smaller groups of CAMs serve as minimal, functionally cooperative adhesive units upon which structure is based. Synapse structure and function evolve over the course of development, and in mature animals, synapses are composed of a greater number of proteins, surrounded by a stabilizing extracellular matrix, and often contacted by astrocytic processes. Thus, in mature networks undergoing plasticity, persistent changes in synapse strength, morphology, or number must be accompanied by selective and regulated remodeling of the neuropil. Recent work indicates that regulated, extracellular proteolysis may be essential for this, and rather than simply acting permissively to enable synapse plasticity, is more likely playing a proactive role in driving coordinated synaptic structural and functional modifications that underlie persistent changes in network activity.
Copyright © 2010 Wiley Periodicals, Inc.
Figures

Similar articles
-
[The role of extracellular proteolysis in synaptic plasticity of the central nervous system].Postepy Hig Med Dosw (Online). 2012 Nov 29;66:959-75. doi: 10.5604/17322693.1021851. Postepy Hig Med Dosw (Online). 2012. PMID: 23687215 Review. Polish.
-
Proteolytic remodeling of the synaptic cell adhesion molecules (CAMs) by metzincins in synaptic plasticity.Neurochem Res. 2013 Jun;38(6):1113-21. doi: 10.1007/s11064-012-0919-6. Epub 2012 Nov 4. Neurochem Res. 2013. PMID: 23124395 Free PMC article. Review.
-
Reelin, lipoprotein receptors and synaptic plasticity.Nat Rev Neurosci. 2006 Nov;7(11):850-9. doi: 10.1038/nrn2009. Nat Rev Neurosci. 2006. PMID: 17053810 Review.
-
Surface traffic in synaptic membranes.Adv Exp Med Biol. 2012;970:197-219. doi: 10.1007/978-3-7091-0932-8_9. Adv Exp Med Biol. 2012. PMID: 22351057 Review.
-
Serine proteases regulating synaptic plasticity.Anat Sci Int. 2004 Sep;79(3):137-44. doi: 10.1111/j.1447-073x.2004.00080.x. Anat Sci Int. 2004. PMID: 15453614 Review.
Cited by
-
Synaptic recognition molecules in development and disease.Curr Top Dev Biol. 2021;142:319-370. doi: 10.1016/bs.ctdb.2020.12.009. Epub 2021 Feb 12. Curr Top Dev Biol. 2021. PMID: 33706921 Free PMC article.
-
A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders.Front Cell Neurosci. 2015 Jul 29;9:280. doi: 10.3389/fncel.2015.00280. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26283917 Free PMC article. Review.
-
Cadherin-based transsynaptic networks in establishing and modifying neural connectivity.Curr Top Dev Biol. 2015;112:415-65. doi: 10.1016/bs.ctdb.2014.11.025. Epub 2015 Feb 11. Curr Top Dev Biol. 2015. PMID: 25733148 Free PMC article. Review.
-
Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic β1-integrin.J Comp Neurol. 2012 Jun 15;520(9):2041-52. doi: 10.1002/cne.23027. J Comp Neurol. 2012. PMID: 22488504 Free PMC article.
-
Structural Components of Synaptic Plasticity and Memory Consolidation.Cold Spring Harb Perspect Biol. 2015 Jul 1;7(7):a021758. doi: 10.1101/cshperspect.a021758. Cold Spring Harb Perspect Biol. 2015. PMID: 26134321 Free PMC article. Review.
References
-
- Akins MR, Biederer T. Cell-cell interactions in synaptogenesis. Curr Opin Neurobiol. 2006;16(1):83–9. - PubMed
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119(2):257–72. - PubMed
-
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long- lasting potentiation. Science. 2001;294(5546):1547–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

