Synthetic antigens representing the antigenic variation of human hepatitis C virus
- PMID: 20883164
- PMCID: PMC4056460
- DOI: 10.1089/vim.2010.0043
Synthetic antigens representing the antigenic variation of human hepatitis C virus
Abstract
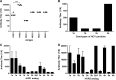
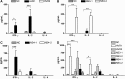
Immune responses against hepatitis C virus (HCV) have been studied by numerous groups. However, details concerning the production of antibodies to antigenically variable epitopes remain to be elucidated. Since the sequences of the variable regions of several HCV proteins are different among the virus strains infecting patients, we decided to design peptide combinations that represent the theoretical maximum antigenic variation of each epitope to be used as capture antigens. We prepared six peptide mixtures (hypervariable epitope constructs; HECs) representing six different epitopes from structural and non-structural proteins of HCV from genotypes 1-6. Plasma from 300 HCV patients was tested to determine if their antibodies recognize the synthetic constructs. All the patients were chronically infected with diverse HCV genotypes and did not receive antiviral treatment. Antibodies to one or more of the HECs were detected in all of the HCV-infected individuals. Immunogenicity of the HCV HECs was also evaluated in outbred and inbred mice. Strong HEC-specific antibodies were produced, and cellular responses were also induced that were Th-1 rather than Th-2. Our results show that HCV HECs are both antigens that can be used to detect the broad cross-reactivity of antibodies from HCV-infected patients, and strong immunogens that can induce antigen-specific humoral and cellular immune responses in mice.
Figures



References
-
- Aberle JH. Formann E. Steindl-Munda P, et al. Prospective study of viral clearance and CD4(+) T-cell response in acute hepatitis C primary infection and reinfection. J Clin Virol. 2006;36:24–31. - PubMed
-
- Anderson DE. Malley A. Benjamini E. Gardner MB. Torres JV. Hypervariable epitope constructs as a means of accounting for epitope variability. Vaccine. 1994;12:736–740. - PubMed
-
- Anderson DE. Singapuri A. Kang KH. Montefiori DC. Torres JV. Timing of retroviral infection influences anamnestic immune response in vaccinated primates. Viral Immunol. 2005;18:689–694. - PubMed
-
- Blum HE. Variants of hepatitis B, C and D viruses: molecular biology and clinical significance. Digestion. 1995;56:85–95. - PubMed
-
- Bowen DG. Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

