Effect of combining extended-release carvedilol and lisinopril in hypertension: results of the COSMOS study
- PMID: 20883227
- PMCID: PMC8673069
- DOI: 10.1111/j.1751-7176.2010.00341.x
Effect of combining extended-release carvedilol and lisinopril in hypertension: results of the COSMOS study
Abstract
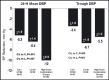
Hypertension treatment commonly requires multiple agents to achieve target blood pressure (BP). β-blockers and angiotensin-converting enzyme inhibitors (ACEIs) are commonly co-prescribed in clinical practice although few data are available that test their additivity on BP lowering. The efficacy and safety of once-daily extended-release carvedilol (carvedilol CR) combined with the ACEI lisinopril in a double-blind, randomized, factorial design study were studied. Patients (N=656) with stage 1 or 2 hypertension were randomized evenly to 1 of 15 groups for 6 weeks: carvedilol CR monotherapy 20 mg, 40 mg, or 80 mg/d; lisinopril monotherapy 10 mg, 20 mg, or 40 mg/d; or 1 of 9 combinations of carvedilol CR plus lisinopril initiated simultaneously. Primary efficacy measures (assessed by ambulatory BP monitoring [ABPM]) were change from baseline in 24-hour mean diastolic BP (DBP) and in trough (20-24 hours) DBP. Continuous efficacy variables were assessed using analysis of covariance. Whether any combination dose was superior to its monotherapy components was assessed using the Hung AVE procedure. Despite the presence of additional BP lowering observed with most of the combinations compared with their monotherapy components, the Hung AVE test was not significant for either primary efficacy measures. Post hoc analyses of the high-dose combination groups (carvedilol CR/lisinopril regimens of 80/10 mg, 80/20 mg, 80/40 mg, 20/40 mg, and 40/40 mg) showed a significant treatment difference compared with both carvedilol CR 80 mg and lisinopril 40 mg for 24-hour mean DBP but not for trough DBP. With the exception of dizziness, individual adverse events did not increase with ascending doses or combinations. The superiority of initiating combination treatment with carvedilol CR and lisinopril compared with the monotherapy components was not demonstrated with the ABPM measurements. Nonetheless, the post hoc assessment combining all high-dose groups did produce significant 24-hour mean BP reduction when compared with the high-dose monotherapy groups. The tolerability profile of initiating combination therapy was generally comparable to the initiation of treatment with monotherapy.
Trial registration: ClinicalTrials.gov NCT00347360.
© 2010 Wiley Periodicals, Inc.
Figures

Comment in
-
Effect of combining extended-release carvedilol and lisinopril in hypertension: results of the COSMOS study.J Clin Hypertens (Greenwich). 2010 Sep;12(9):664-5. doi: 10.1111/j.1751-7176.2010.00351.x. J Clin Hypertens (Greenwich). 2010. PMID: 20928961 Free PMC article. Clinical Trial. No abstract available.
References
-
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. - PubMed
-
- Bakris GL. An approach to achieving recommended blood pressure goals in diabetic patients. Arch Intern Med. 2001;161:2661–2667. - PubMed
-
- Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benzazeprilHCl versus comparable component‐based therapy. Chem Herit. 2003;9:324–332. - PubMed
-
- Jamerson K, Weber MA, Bakris GL, et al.; ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008. ;359:2417–2428 - PubMed
-
- Weinberger MH. Blood pressure and metabolic responses to hydrochlorothiazide, captopril, and the combination in black and white mild‐to‐moderate hypertensive patients. J Cardiovasc Pharmacol. 1985;7(suppl 1):S22–S55. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

