Lubiprostone targets prostanoid EP₄ receptors in ovine airways
- PMID: 20883477
- PMCID: PMC3031069
- DOI: 10.1111/j.1476-5381.2010.01058.x
Lubiprostone targets prostanoid EP₄ receptors in ovine airways
Abstract
Background and purpose: Lubiprostone, a prostaglandin E₁ derivative, is reported to activate ClC-2 chloride channels located in the apical membranes of a number of transporting epithelia. Lack of functioning CFTR chloride channels in epithelia is responsible for the genetic disease cystic fibrosis, therefore, surrogate channels that can operate independently of CFTR are of interest. This study explores the target receptor(s) for lubiprostone in airway epithelium.
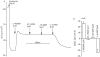
Experimental approach: All experiments were performed on the ventral tracheal epithelium of sheep. Epithelia were used to measure anion secretion from the apical surface as short circuit current or as fluid secretion from individual airway submucosal glands, using an optical method.
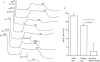
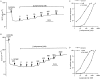
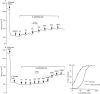
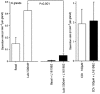
Key results: The EP₄ antagonists L-161982 and GW627368 inhibited short circuit current responses to lubiprostone, while EP₁(,)₂(&)₃ receptor antagonists were without effect. Similarly, lubiprostone induced secretion in airway submucosal glands was inhibited by L-161982. L-161982 effectively competed with lubiprostone with a K(d) value of 0.058 µM, close to its value for binding to human EP₄ receptors (0.024 µM). The selective EP₄ agonist L-902688 and lubiprostone behaved similarly with respect to EP₄ receptor antagonists. Results of experiments with H89, a protein kinase A inhibitor, were consistent with lubiprostone acting through a G(s) -protein coupled EP₄ receptor/cAMP cascade.
Conclusions and implications: Lubiprostone-induced short-circuit currents and submucosal gland secretions were inhibited by selective EP₄ receptor antagonists. The results suggest EP₄ receptor activation by lubiprostone triggers cAMP production necessary for CFTR activation and the secretory responses, a possibility precluded in CF tissues.
© 2010 The Author. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures
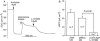
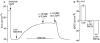

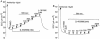

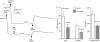
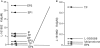
Similar articles
-
Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator.Gastroenterology. 2009 Sep;137(3):976-85. doi: 10.1053/j.gastro.2009.05.037. Epub 2009 May 18. Gastroenterology. 2009. PMID: 19454284
-
Lubiprostone activates CFTR, but not ClC-2, via the prostaglandin receptor (EP(4)).Biochem Biophys Res Commun. 2012 Sep 28;426(3):374-9. doi: 10.1016/j.bbrc.2012.08.097. Epub 2012 Aug 29. Biochem Biophys Res Commun. 2012. PMID: 22960173 Free PMC article.
-
Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR.Br J Pharmacol. 2007 Apr;150(8):1055-65. doi: 10.1038/sj.bjp.0707175. Epub 2007 Mar 5. Br J Pharmacol. 2007. PMID: 17339840 Free PMC article.
-
HCO3- transport in relation to mucus secretion from submucosal glands.JOP. 2001 Jul;2(4 Suppl):280-4. JOP. 2001. PMID: 11875272 Review.
-
Anoctamin 1 in secretory epithelia.Cell Calcium. 2014 Jun;55(6):355-61. doi: 10.1016/j.ceca.2014.02.006. Epub 2014 Feb 15. Cell Calcium. 2014. PMID: 24636668 Review.
Cited by
-
Effects of lubiprostone on pacemaker activity of interstitial cells of cajal from the mouse colon.Korean J Physiol Pharmacol. 2014 Aug;18(4):341-6. doi: 10.4196/kjpp.2014.18.4.341. Epub 2014 Aug 13. Korean J Physiol Pharmacol. 2014. PMID: 25177167 Free PMC article.
-
Clubbed Digits Presumably Caused by Lubiprostone.Intern Med. 2021;60(15):2499-2502. doi: 10.2169/internalmedicine.6104-20. Epub 2021 Aug 1. Intern Med. 2021. PMID: 34334594 Free PMC article.
-
Receptor-mediated activation of CFTR via prostaglandin signaling pathways in the airway.Am J Physiol Lung Cell Mol Physiol. 2022 Mar 1;322(3):L305-L314. doi: 10.1152/ajplung.00388.2021. Epub 2022 Jan 12. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 35020527 Free PMC article.
-
May the truth be with you: lubiprostone as EP receptor agonist/ClC-2 internalizing "inhibitor".Dig Dis Sci. 2012 Nov;57(11):2740-2. doi: 10.1007/s10620-012-2410-2. Dig Dis Sci. 2012. PMID: 23001408 No abstract available.
-
Lubiprostone is non-selective activator of cAMP-gated ion channels and Clc-2 has a minor role in its prosecretory effect in intestinal epithelial cells.Mol Pharmacol. 2022 Jun 9;102(2):106-15. doi: 10.1124/molpharm.122.000542. Mol Pharmacol. 2022. PMID: 35680165 Free PMC article.
References
-
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Phamacol. 2009;158:S1–S254.
-
- Ballard ST, Trout L, Bebok Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol. 1999;277:L694–L699. - PubMed
-
- Bijvelds MJC, Bot AGM, Escher J, De Jonge HR. Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–985. - PubMed
-
- Billot X, Chateauneuf A, Chauret N, Denis D, Greig GM, Mathieu M-C, et al. Discovery of a highly potent and selective agonist of the prostaglandin EP4 receptor. Bioorg Med Chem Lett. 2003;13:1129–1132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

