B cells help alloreactive T cells differentiate into memory T cells
- PMID: 20883532
- PMCID: PMC2956128
- DOI: 10.1111/j.1600-6143.2010.03223.x
B cells help alloreactive T cells differentiate into memory T cells
Abstract
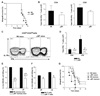
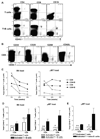
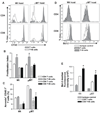
B cells are recognized as effector cells in allograft rejection that are dependent upon T cell help to produce alloantibodies causing graft injury. It is not known if B cells can also help T cells differentiate into memory cells in the alloimmune response. We found that in B-cell-deficient hosts, differentiation of alloreactive T cells into effectors was intact whereas their development into memory T cells was impaired. To test if B cell help for T cells was required for their continued differentiation into memory T cells, activated T cells were sorted from alloimmunized mice and transferred either with or without B cells into naïve adoptive hosts. Activated T cells cotransferred with B cells gave rise to more memory T cells than those transferred without B cells and upon recall, mediated accelerated rejection of skin allografts. Cotransfer of B cells led to increased memory T cells by enhancing activated CD4 T-cell proliferation and activated CD8 T-cell survival. These results indicate that B cells help alloreactive T-cell differentiation, proliferation and survival to generate optimal numbers of functional memory T cells.
© 2010 The Authors Journal compilation © 2010 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures


Similar articles
-
Alloreactive CD8 T-cell primed/memory responses and accelerated graft rejection in B-cell-deficient sensitized mice.Transplantation. 2011 May 27;91(10):1075-81. doi: 10.1097/TP.0b013e31821578da. Transplantation. 2011. PMID: 21427633 Free PMC article.
-
Rapamycin or tacrolimus alone fails to resist cardiac allograft accelerated rejection mediated by alloreactive CD4(+) memory T cells in mice.Transpl Immunol. 2010 Feb;22(3-4):128-36. doi: 10.1016/j.trim.2009.09.003. Epub 2009 Sep 13. Transpl Immunol. 2010. PMID: 19755159
-
Role of regulated upon activation normal T-cell expressed and secreted in a model of retransplantation acute rejection mediated by alloreactive memory CD4+ T cells.Transplant Proc. 2013 Mar;45(2):546-51. doi: 10.1016/j.transproceed.2012.11.005. Transplant Proc. 2013. PMID: 23498790
-
T cells and memory lapses.Trends Microbiol. 1997 Jul;5(7):259-60; discussion 260-1. doi: 10.1016/S0966-842X(97)01072-X. Trends Microbiol. 1997. PMID: 9234501 Review. No abstract available.
-
The Entangled World of Memory T Cells and Implications in Transplantation.Transplantation. 2024 Jan 1;108(1):137-147. doi: 10.1097/TP.0000000000004647. Epub 2023 Dec 13. Transplantation. 2024. PMID: 37271872 Free PMC article. Review.
Cited by
-
CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity.Am J Transplant. 2013 Nov;13(11):2831-2841. doi: 10.1111/ajt.12432. Epub 2013 Sep 18. Am J Transplant. 2013. PMID: 24102790 Free PMC article.
-
Immunomodulatory effect of mesenchymal stem cells on B cells.Front Immunol. 2012 Jul 20;3:212. doi: 10.3389/fimmu.2012.00212. eCollection 2012. Front Immunol. 2012. PMID: 22833744 Free PMC article.
-
Immune recognition and rejection of allogeneic skin grafts.Immunotherapy. 2011 Jun;3(6):757-70. doi: 10.2217/imt.11.2. Immunotherapy. 2011. PMID: 21668313 Free PMC article. Review.
-
B-cell-dependent memory T cells impede nonmyeloablative mixed chimerism induction in presensitized mice.Am J Transplant. 2011 Nov;11(11):2322-31. doi: 10.1111/j.1600-6143.2011.03683.x. Epub 2011 Aug 10. Am J Transplant. 2011. PMID: 21831158 Free PMC article.
-
IRF4 ablation in B cells abrogates allogeneic B cell responses and prevents chronic transplant rejection.J Heart Lung Transplant. 2021 Oct;40(10):1122-1132. doi: 10.1016/j.healun.2021.06.008. Epub 2021 Jun 23. J Heart Lung Transplant. 2021. PMID: 34253454 Free PMC article.
References
-
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. - PubMed
-
- Rosenberg AS. Skin Allograft Rejection. Hoboken, NJ: John Wiley & Sons, Inc.; 1991.
-
- Obhrai J, Oberbarnscheidt M, Hand T, Diggs L, Chalasani G, Lakkis F. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. - PubMed
-
- Barber D, Wherry E, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. - PubMed
-
- Papadopoulos N, Dedoussis G, Spanakos G, Gritzapis A, Baxevanis C, Papamichail M. An improved fluorescence assay for he determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177:101–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

