Cord blood administration induces oligodendrocyte survival through alterations in gene expression
- PMID: 20883670
- PMCID: PMC2993822
- DOI: 10.1016/j.brainres.2010.09.078
Cord blood administration induces oligodendrocyte survival through alterations in gene expression
Abstract
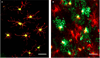
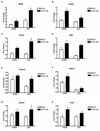
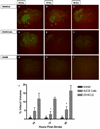
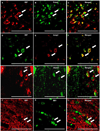
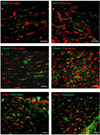
Oligodendrocytes (OLs), the predominant cell type found in cerebral white matter, are essential for structural integrity and proper neural signaling. Very little is known concerning stroke-induced OL dysfunction. Our laboratory has shown that infusion of human umbilical cord blood (HUCB) cells protects striatal white matter tracts in vivo and directly protects mature primary OL cultures from oxygen glucose deprivation (OGD). Microarray studies of RNA prepared from OL cultures subjected to OGD and treated with HUCB cells showed an increase in the expression of 33 genes associated with OL proliferation, survival, and repair functions, such as myelination. The microarray results were verified using quantitative RT-PCR for the following eight genes: U2AF homology motif kinase 1 (Uhmk1), insulin-induced gene 1 (Insig1), metallothionein 3 (Mt3), tetraspanin 2 (Tspan2), peroxiredoxin 4 (Prdx4), stathmin-like 2 (Stmn2), myelin oligodendrocyte glycoprotein (MOG), and versican (Vcan). Immunohistochemistry showed that MOG, Prdx4, Uhmk1, Insig1, and Mt3 protein expression were upregulated in the ipsilateral white matter tracts of rats infused with HUCB cells 48h after middle cerebral artery occlusion (MCAO). Furthermore, promoter region analysis of these genes revealed common transcription factor binding sites, providing insight into the shared signal transduction pathways activated by HUCB cells to enhance transcription of these genes. These results show expression of genes induced by HUCB cell therapy that could confer oligoprotection from ischemia.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia.Eur J Neurosci. 2014 Oct;40(7):3111-9. doi: 10.1111/ejn.12675. Epub 2014 Jul 16. Eur J Neurosci. 2014. PMID: 25041106 Free PMC article.
-
Human umbilical cord blood cells induce neuroprotective change in gene expression profile in neurons after ischemia through activation of Akt pathway.Cell Transplant. 2015;24(4):721-35. doi: 10.3727/096368914X685311. Epub 2015 Feb 26. Cell Transplant. 2015. PMID: 25413246 Free PMC article.
-
Effects of hypothermia on oligodendrocyte precursor cell proliferation, differentiation and maturation following hypoxia ischemia in vivo and in vitro.Exp Neurol. 2013 Sep;247:720-9. doi: 10.1016/j.expneurol.2013.03.015. Epub 2013 Mar 22. Exp Neurol. 2013. PMID: 23524193
-
Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination.Neuron. 2010 Mar 11;65(5):597-611. doi: 10.1016/j.neuron.2010.01.027. Neuron. 2010. PMID: 20223197 Free PMC article.
-
Human umbilical cord blood cells protect oligodendrocytes from brain ischemia through Akt signal transduction.J Biol Chem. 2012 Feb 3;287(6):4177-87. doi: 10.1074/jbc.M111.296434. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158864 Free PMC article.
Cited by
-
Stem cell-based interventions for the prevention and treatment of germinal matrix-intraventricular haemorrhage in preterm infants.Cochrane Database Syst Rev. 2019 Sep 24;9(9):CD013201. doi: 10.1002/14651858.CD013201.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Feb 15;2:CD013201. doi: 10.1002/14651858.CD013201.pub3. PMID: 31549743 Free PMC article. Updated.
-
Leukemia Inhibitory Factor Receptor Is Involved in Apoptosis in Rat Astrocytes Exposed to Oxygen-Glucose Deprivation.Biomed Res Int. 2019 Feb 27;2019:1613820. doi: 10.1155/2019/1613820. eCollection 2019. Biomed Res Int. 2019. PMID: 30937308 Free PMC article.
-
Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia.Eur J Neurosci. 2014 Oct;40(7):3111-9. doi: 10.1111/ejn.12675. Epub 2014 Jul 16. Eur J Neurosci. 2014. PMID: 25041106 Free PMC article.
-
Plasticity of the myelination genomic fabric.Mol Genet Genomics. 2012 Mar;287(3):237-46. doi: 10.1007/s00438-012-0673-0. Epub 2012 Jan 13. Mol Genet Genomics. 2012. PMID: 22246408
-
Fetal Neuroprotective Mechanism of Maternal Magnesium Sulfate: Proteomic Analysis.J Mol Neurosci. 2022 Mar;72(3):626-632. doi: 10.1007/s12031-021-01939-y. Epub 2021 Nov 10. J Mol Neurosci. 2022. PMID: 34761370
References
-
- Baron-Van Evercooren A, Olichon-Berthe C, Kowalski A, Visciano G, Van Obberghen E. Expression of IGF-I and insulin receptor genes in the rat central nervous system: a developmental, regional, and cellular analysis. J Neurosci Res. 1991;28:244–253. - PubMed
-
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
-
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. - PubMed
-
- Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

