In vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in mice
- PMID: 20883755
- PMCID: PMC2982888
- DOI: 10.1016/j.ijpharm.2010.09.015
In vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in mice
Abstract
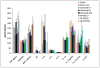
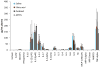
Porous silicon (pSi) is being extensively studied as an emerging material for use in biomedical applications, including drug delivery, based on the biodegradability and versatile chemical and biophysical properties. We have recently introduced multistage nanoporous silicon microparticles (S1MP) designed as a cargo for nanocarrier drug delivery to enable the loaded therapeutics and diagnostics to sequentially overcome the biological barriers in order to reach their target. In this first report on biocompatibility of intravenously administered pSi structures, we examined the tolerability of negatively (-32.5±3.1mV) and positively (8.7±2.5mV) charged S1MP in acute single dose (10(7), 10(8), 5×10(8) S1MP/animal) and subchronic multiple dose (10(8) S1MP/animal/week for 4 weeks) administration schedules. Our data demonstrate that S1MP did not change plasma levels of renal (BUN and creatinine) and hepatic (LDH) biomarkers as well as 23 plasma cytokines. LDH plasma levels of 145.2±23.6, 115.4±29.1 vs. 127.0±10.4; and 155.8±38.4, 135.5±52.3 vs. 178.4±74.6 were detected in mice treated with 10(8) negatively charged S1MP, 10(8) positively charged S1MP vs. saline control in single and multiple dose schedules, respectively. The S1MPs did not alter LDH levels in liver and spleen, nor lead to infiltration of leukocytes into the liver, spleen, kidney, lung, brain, heart, and thyroid. Collectively, these data provide evidence of a safe intravenous administration of S1MPs as a drug delivery carrier.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Cytotoxicity assessment of porous silicon microparticles for ocular drug delivery.Eur J Pharm Biopharm. 2016 Mar;100:1-8. doi: 10.1016/j.ejpb.2015.11.020. Epub 2015 Dec 11. Eur J Pharm Biopharm. 2016. PMID: 26686646
-
Investigation of silicon nanoparticles produced by centrifuge chemical vapor deposition for applications in therapy and diagnostics.Eur J Pharm Biopharm. 2021 Jan;158:254-265. doi: 10.1016/j.ejpb.2020.11.022. Epub 2020 Dec 3. Eur J Pharm Biopharm. 2021. PMID: 33279602
-
Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics.Acc Chem Res. 2011 Oct 18;44(10):979-89. doi: 10.1021/ar200077p. Epub 2011 Sep 8. Acc Chem Res. 2011. PMID: 21902173 Free PMC article. Review.
-
Design and in vitro characterization of multistage silicon-PLGA budesonide particles for inflammatory bowel disease.Eur J Pharm Biopharm. 2020 Jun;151:61-72. doi: 10.1016/j.ejpb.2020.03.020. Epub 2020 Apr 10. Eur J Pharm Biopharm. 2020. PMID: 32283213
-
Multifunctional porous silicon for therapeutic drug delivery and imaging.Curr Drug Discov Technol. 2011 Sep;8(3):228-49. doi: 10.2174/157016311796799053. Curr Drug Discov Technol. 2011. PMID: 21291407 Review.
Cited by
-
Modeling of Nanotherapy Response as a Function of the Tumor Microenvironment: Focus on Liver Metastasis.Front Bioeng Biotechnol. 2020 Aug 19;8:1011. doi: 10.3389/fbioe.2020.01011. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974325 Free PMC article. Review.
-
Post-nano strategies for drug delivery: Multistage porous silicon microvectors.J Mater Chem B. 2017 Jan 14;5(2):207-219. doi: 10.1039/C6TB01978A. Epub 2016 Nov 26. J Mater Chem B. 2017. PMID: 28670454 Free PMC article.
-
Safety of poly (ethylene glycol)-coated perfluorodecalin-filled poly (lactide-co-glycolide) microcapsules following intravenous administration of high amounts in rats.Results Pharma Sci. 2014 Apr 30;4:8-18. doi: 10.1016/j.rinphs.2014.04.001. eCollection 2014. Results Pharma Sci. 2014. PMID: 25756002 Free PMC article.
-
Enhanced performance of macrophage-encapsulated nanoparticle albumin-bound-paclitaxel in hypo-perfused cancer lesions.Nanoscale. 2016 Jul 7;8(25):12544-52. doi: 10.1039/c5nr07796f. Epub 2016 Jan 28. Nanoscale. 2016. PMID: 26818212 Free PMC article.
-
Multifunctional to multistage delivery systems: The evolution of nanoparticles for biomedical applications.Chin Sci Bull. 2012 Nov 1;57(31):3961-3971. doi: 10.1007/s11434-012-5387-5. Chin Sci Bull. 2012. PMID: 24587616 Free PMC article.
References
-
- Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–199. - PubMed
-
- Canham LT. Bioactive silicon structure fabrication through nanoetching techniques. Advanced Materials. 1995a;7:1033–1037.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

