Emergence of the acute-phase protein hemopexin in jawed vertebrates
- PMID: 20884052
- PMCID: PMC2993785
- DOI: 10.1016/j.molimm.2010.08.015
Emergence of the acute-phase protein hemopexin in jawed vertebrates
Abstract
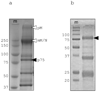
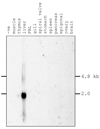
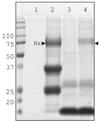
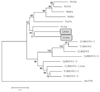
When released from damaged erythrocytes free heme not only provides a source of iron for invading bacteria but also highly toxic due to its ability to catalyze free radical formation. Hemopexin (Hx) binds free heme with very high-affinity and thus protects against heme toxicity, sequesters heme from pathogens, and helps conserve valuable iron. Hx is also an acute-phase serum protein (APP), whose expression is induced by inflammation. To date Hx has been identified as far back in phylogeny as bony fish where it is called warm-temperature acclimation-related 65 kDa protein (WAP65), as serum protein levels are increased at elevated environmental temperatures as well as by infection. During analysis of nurse shark (Ginglymostoma cirratum) plasma we isolated a Ni(2+)-binding serum glycoprotein and characterized it as the APP Hx. We subsequently cloned Hx from nurse shark and another cartilaginous fish species, the little skate Leucoraja erinacea. Functional analysis showed shark Hx, like that of mammals, binds heme but is found at unusually high levels in normal shark serum. As an Hx orthologue could not be found in the genomes of jawless vertebrates or lower deuterostomes it appears to have arisen just prior to the emergence of jawed vertebrates, coincident with the second round of genome-wide duplication and the appearance of tetrameric hemoglobin (Hb).
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Adaptive functional divergence of the warm temperature acclimation-related protein (WAP65) in fishes and the ortholog hemopexin (HPX) in mammals.J Hered. 2014 Mar-Apr;105(2):237-52. doi: 10.1093/jhered/est087. Epub 2013 Dec 16. J Hered. 2014. PMID: 24344252
-
A tale of two genes: divergent evolutionary fate of haptoglobin and hemopexin in hemoglobinless Antarctic icefishes.J Exp Biol. 2019 Mar 21;222(Pt 6):jeb188573. doi: 10.1242/jeb.188573. J Exp Biol. 2019. PMID: 30765469
-
The warm temperature acclimation protein (Wap65) has an important role in the inflammatory response of turbot (Scophthalmus maximus).Fish Shellfish Immunol. 2014 Nov;41(1):80-92. doi: 10.1016/j.fsi.2014.04.012. Epub 2014 Apr 30. Fish Shellfish Immunol. 2014. PMID: 24794581
-
Big insight from the little skate: Leucoraja erinacea as a developmental model system.Curr Top Dev Biol. 2022;147:595-630. doi: 10.1016/bs.ctdb.2021.12.016. Epub 2022 Feb 28. Curr Top Dev Biol. 2022. PMID: 35337464 Review.
-
Evidence for Sleep in Sharks and Rays: Behavioural, Physiological, and Evolutionary Considerations.Brain Behav Evol. 2019;94(1-4):37-50. doi: 10.1159/000504123. Epub 2019 Nov 27. Brain Behav Evol. 2019. PMID: 31775150 Review.
Cited by
-
Nutritional Immunity Triggers the Modulation of Iron Metabolism Genes in the Sub-Antarctic Notothenioid Eleginops maclovinus in Response to Piscirickettsia salmonis.Front Immunol. 2017 Sep 19;8:1153. doi: 10.3389/fimmu.2017.01153. eCollection 2017. Front Immunol. 2017. PMID: 28974951 Free PMC article.
-
Exploration of the Nurse Shark (Ginglymostoma cirratum) Plasma Immunoproteome Using High-Resolution LC-MS/MS.Front Immunol. 2022 Jun 6;13:873390. doi: 10.3389/fimmu.2022.873390. eCollection 2022. Front Immunol. 2022. PMID: 35734164 Free PMC article.
-
Ancient Use of Ig Variable Domains Contributes Significantly to the TCRδ Repertoire.J Immunol. 2019 Sep 1;203(5):1265-1275. doi: 10.4049/jimmunol.1900369. Epub 2019 Jul 24. J Immunol. 2019. PMID: 31341077 Free PMC article.
-
Involvement of apolipoprotein A in maintaining tissue fluid balance in goldfish Carassius auratus.Fish Physiol Biochem. 2019 Oct;45(5):1717-1730. doi: 10.1007/s10695-019-00662-1. Epub 2019 Jun 21. Fish Physiol Biochem. 2019. PMID: 31227941
-
Dietary Yeast Cell Wall Extract Alters the Proteome of the Skin Mucous Barrier in Atlantic Salmon (Salmo salar): Increased Abundance and Expression of a Calreticulin-Like Protein.PLoS One. 2017 Jan 3;12(1):e0169075. doi: 10.1371/journal.pone.0169075. eCollection 2017. PLoS One. 2017. PMID: 28046109 Free PMC article.
References
-
- Bartl S, Baish MA, Flajnik MF, Ohta Y. Identification of class I genes in cartilaginous fish, the most ancient group of vertebrates displaying an adaptive immune response. J. Immunol. 1997;159:6097–6104. - PubMed
-
- Blair JE, Hedges SB. Molecular phylogeny and divergence times of deuterostome animals. Mol. Biol. Evol. 2005;22:2275–2284. - PubMed
-
- Coates ML. Hemoglobin function in the vertebrates: an evolutionary model. J. Mol. Evol. 1975;6:285–307. - PubMed
-
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

