Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation
- PMID: 20884629
- PMCID: PMC2982943
- DOI: 10.1158/0008-5472.CAN-10-1854
Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation
Abstract
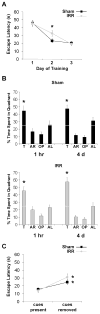
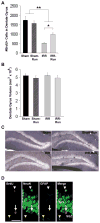
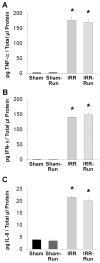
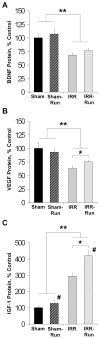
Whole-brain irradiation (WBI) therapy produces progressive learning and memory deficits in patients with primary or secondary brain tumors. Exercise enhances memory and adult hippocampal neurogenesis in the intact brain, so we hypothesized that exercise may be an effective treatment to alleviate consequences of WBI. Previous studies using animal models to address this issue have yielded mixed results and have not examined potential molecular mechanisms. We investigated the short- and long-term effects of WBI on spatial learning and memory retention and determined whether voluntary running after WBI aids recovery of brain and cognitive function. Forty adult female C57Bl/6 mice given a single dose of 5 Gy or sham WBI were trained 2.5 weeks and up to 4 months after WBI in a Barnes maze. Half of the mice received daily voluntary wheel access starting 1 month after sham or WBI. Daily running following WBI prevented the marked decline in spatial memory retention observed months after irradiation. Bromodeoxyuridine (BrdUrd) immunolabeling and enzyme-linked immunosorbent assay indicated that this behavioral rescue was accompanied by a partial restoration of newborn BrdUrd+/NeuN+ neurons in the dentate gyrus and increased hippocampal expression of brain-derived vascular endothelial growth factor and insulin-like growth factor-1, and occurred despite irradiation-induced elevations in hippocampal proinflammatory cytokines. WBI in adult mice produced a progressive memory decline consistent with what has been reported in cancer patients receiving WBI therapy. Our findings show that running can abrogate this memory decline and aid recovery of adult hippocampal plasticity, thus highlighting exercise as a potential therapeutic intervention.
Copyright © 2010 AACR.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures

Similar articles
-
The PPARδ agonist GW0742 inhibits neuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation.Free Radic Biol Med. 2013 Aug;61:1-9. doi: 10.1016/j.freeradbiomed.2013.03.002. Epub 2013 Mar 14. Free Radic Biol Med. 2013. PMID: 23499837 Free PMC article.
-
Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice.Neurobiol Learn Mem. 2016 May;131:26-35. doi: 10.1016/j.nlm.2016.03.002. Epub 2016 Mar 8. Neurobiol Learn Mem. 2016. PMID: 26968656
-
Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway.Biochem Biophys Res Commun. 2014 Jan 10;443(2):646-51. doi: 10.1016/j.bbrc.2013.12.031. Epub 2013 Dec 11. Biochem Biophys Res Commun. 2014. PMID: 24333433
-
Mechanisms underlying the effect of voluntary running on adult hippocampal neurogenesis.Hippocampus. 2023 Apr;33(4):373-390. doi: 10.1002/hipo.23520. Epub 2023 Mar 9. Hippocampus. 2023. PMID: 36892196 Free PMC article. Review.
-
All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis.Curr Top Behav Neurosci. 2013;15:189-210. doi: 10.1007/7854_2012_220. Curr Top Behav Neurosci. 2013. PMID: 22847651 Free PMC article. Review.
Cited by
-
Delayed voluntary exercise does not enhance cognitive performance after hippocampal injury: an investigation of differentially distributed exercise protocols.J Exerc Rehabil. 2016 Oct 31;12(5):401-412. doi: 10.12965/jer.1632680.340. eCollection 2016 Oct. J Exerc Rehabil. 2016. PMID: 27807517 Free PMC article.
-
Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms.Neuro Oncol. 2014 Apr;16(4):476-92. doi: 10.1093/neuonc/not321. Epub 2014 Jan 26. Neuro Oncol. 2014. PMID: 24470543 Free PMC article. Review.
-
Impact of Radiation Therapy Techniques on Hippocampal Doses and Psychological Status in Patients With Nasopharyngeal Carcinoma.Cancer Manag Res. 2025 Jan 16;17:83-90. doi: 10.2147/CMAR.S492449. eCollection 2025. Cancer Manag Res. 2025. PMID: 39834795 Free PMC article.
-
Relationship between exercise behavior, cardiorespiratory fitness, and cognitive function in early breast cancer patients treated with doxorubicin-containing chemotherapy: a pilot study.Appl Physiol Nutr Metab. 2014 Jun;39(6):724-9. doi: 10.1139/apnm-2013-0380. Epub 2013 Dec 3. Appl Physiol Nutr Metab. 2014. PMID: 24869976 Free PMC article.
-
New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks.Hippocampus. 2012 Sep;22(9):1860-7. doi: 10.1002/hipo.22020. Epub 2012 Mar 30. Hippocampus. 2012. PMID: 22467337 Free PMC article.
References
-
- Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–65. - PubMed
-
- Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14:238–42. - PubMed
-
- Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–8. - PubMed
-
- Marko NF, Weil RJ. Radiotherapy: Neurocognitive considerations in the treatment of brain metastases. Nat Rev Clin Oncol. 2010;7:185–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

