Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: cardiac iron and function comparison determined by quantitative magnetic resonance imaging
- PMID: 20884710
- PMCID: PMC3012763
- DOI: 10.3324/haematol.2009.019042
Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: cardiac iron and function comparison determined by quantitative magnetic resonance imaging
Abstract
Background: Oral deferiprone was suggested to be more effective than subcutaneous desferrioxamine for removing heart iron. Oral once-daily chelator deferasirox has recently been made commercially available but its long-term efficacy on cardiac iron and function has not yet been established. Our study aimed to compare the effectiveness of deferasirox, deferiprone and desferrioxamine on myocardial and liver iron concentrations and bi-ventricular function in thalassemia major patients by means of quantitative magnetic resonance imaging.
Design and methods: From the first 550 thalassemia subjects enrolled in the Myocardial Iron Overload in Thalassemia network, we retrospectively selected thalassemia major patients who had been receiving one chelator alone for longer than one year. We identified three groups of patients: 24 treated with deferasirox, 42 treated with deferiprone and 89 treated with desferrioxamine. Myocardial iron concentrations were measured by T2* multislice multiecho technique. Biventricular function parameters were quantitatively evaluated by cine images. Liver iron concentrations were measured by T2* multiecho technique.
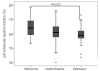
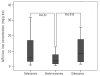
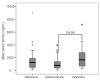
Results: The global heart T2* value was significantly higher in the deferiprone (34 ± 11 ms) than in the deferasirox (21 ± 12 ms) and the desferrioxamine groups (27 ± 11 ms) (P = 0.0001). We found higher left ventricular ejection fractions in the deferiprone and the desferrioxamine versus the deferasirox group (P = 0.010). Liver iron concentration, measured as T2* signal, was significantly lower in the desferrioxamine versus the deferiprone and the deferasirox group (P = 0.004).
Conclusions: The cohort of patients treated with oral deferiprone showed less myocardial iron burden and better global systolic ventricular function compared to the patients treated with oral deferasirox or subcutaneous desferrioxamine.
Figures

Comment in
-
In search of the optimal iron chelation therapy for patients with thalassemia major.Haematologica. 2011 Jan;96(1):5-8. doi: 10.3324/haematol.2010.034397. Haematologica. 2011. PMID: 21193427 Free PMC article. No abstract available.
-
Deferiprone or deferasirox for cardiac siderosis in beta-thalassemia major.Haematologica. 2011 Feb;96(2):e5-6; author reply e7-8. doi: 10.3324/haematol.2010.036061. Haematologica. 2011. PMID: 21282714 Free PMC article. No abstract available.
References
-
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–93. - PubMed
-
- Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Improving survival with deferiprone treatment in patients with thalassemia major: a prospective multicenter randomised clinical trial under the auspices of the Italian Society for Thalassemia and Hemoglobinopathies. Blood Cells Mol Dis. 2009;42(3):247–51. - PubMed
-
- Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in β-thalassaemia. Lancet. 2002;360(9332):516–20. - PubMed
-
- Pepe A, Lombardi M, Positano V, Cracolici E, Capra M, Malizia R, et al. Evaluation of the efficacy of oral deferiprone in β-thalassemia major by multislice multiecho T2*. Eur J Haematol. 2006;76(3):183–92. - PubMed
-
- Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, et al. Randomized controlled trial of deferiprone or deferoxamine in β-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107(9):3738–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

