Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor
- PMID: 20884761
- PMCID: PMC2997037
- DOI: 10.1152/jn.00491.2010
Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor
Abstract
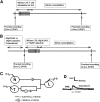
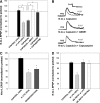
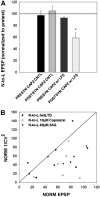
Recent studies have found that some forms of endocannabinoid-dependent synaptic plasticity in the hippocampus are mediated through activation of transient potential receptor vanilloid (TRPV) receptors instead of cannabinoid receptors CB1 or CB2. The potential role for synaptic localization of TRPV receptors during endocannabinoid modulation of nociceptive synapses was examined in the leech CNS where it is possible to record from the same pair of neurons from one preparation to the next. Long-term depression (LTD) in the monosynaptic connection between the nociceptive (N) sensory neuron and the longitudinal (L) motor neuron was found to be endocannabinoid-dependent given that this depression was blocked by RHC-80267, an inhibitor of DAG lipase that is required for 2-arachidonoyl glycerol (2AG) synthesis. Intracellular injection of a second DAG lipase inhibitor, tetrahyrdolipstatin (THL) was also able to block this endocannabinoid-dependent LTD (ecLTD) when injected postsynaptically but not presynaptically. N-to-L ecLTD was also inhibited by the TRPV1 antagonists capsazepine and SB 366791. Bath application of 2AG or the TRPV1 agonists capsaicin and resiniferatoxin mimicked LTD and both capsaicin- and 2AG-induced depression were blocked by capsazepine. In addition, pretreatment with 2AG or capsaicin occluded subsequent expression of LTD induced by repetitive activity. Presynaptic, but not postsynaptic, intracellular injection of capsazepine blocked both activity- and 2AG-induced ecLTD, suggesting that a presynaptic TRPV-like receptor in the leech mediated this form of synaptic plasticity. These findings potentially extend the role ecLTD to nociceptive synapses and suggest that invertebrate synapses, which are thought to lack CB1/CB2 receptor orthologues, utilize a TRPV-like protein as an endocannabinoid receptor.
Figures
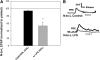

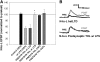
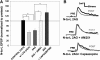

Similar articles
-
Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism.J Neurophysiol. 2013 Dec;110(11):2607-16. doi: 10.1152/jn.00170.2013. Epub 2013 Sep 11. J Neurophysiol. 2013. PMID: 24027102 Free PMC article.
-
Long-term depression of nociceptive synapses by non-nociceptive afferent activity: role of endocannabinoids, Ca²+, and calcineurin.Brain Res. 2012 Jun 15;1460:1-11. doi: 10.1016/j.brainres.2012.04.030. Epub 2012 Apr 24. Brain Res. 2012. PMID: 22578358
-
Endocannabinoid-dependent long-term depression in a nociceptive synapse requires coordinated presynaptic and postsynaptic transcription and translation.J Neurosci. 2013 Mar 6;33(10):4349-58. doi: 10.1523/JNEUROSCI.3922-12.2013. J Neurosci. 2013. PMID: 23467351 Free PMC article.
-
Control of synaptic function by endocannabinoid-mediated retrograde signaling.Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(7):235-50. doi: 10.2183/pjab.90.235. Proc Jpn Acad Ser B Phys Biol Sci. 2014. PMID: 25169670 Free PMC article. Review.
-
Endocannabinoid-mediated synaptic plasticity in the CNS.Annu Rev Neurosci. 2006;29:37-76. doi: 10.1146/annurev.neuro.29.051605.112834. Annu Rev Neurosci. 2006. PMID: 16776579 Review.
Cited by
-
Characterization of a Fatty Acid Amide Hydrolase (FAAH) in Hirudo Verbana.Neurochem Res. 2024 Nov;49(11):3015-3029. doi: 10.1007/s11064-024-04216-7. Epub 2024 Aug 2. Neurochem Res. 2024. PMID: 39093361 Free PMC article.
-
Comparative biology of pain: What invertebrates can tell us about how nociception works.J Neurophysiol. 2017 Apr 1;117(4):1461-1473. doi: 10.1152/jn.00600.2016. Epub 2017 Jan 4. J Neurophysiol. 2017. PMID: 28053241 Free PMC article. Review.
-
Endocannabinoid-mediated potentiation of nonnociceptive synapses contributes to behavioral sensitization.J Neurophysiol. 2018 Feb 1;119(2):641-651. doi: 10.1152/jn.00092.2017. Epub 2017 Nov 8. J Neurophysiol. 2018. PMID: 29118192 Free PMC article.
-
Functional diversity on synaptic plasticity mediated by endocannabinoids.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3242-53. doi: 10.1098/rstb.2011.0386. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108543 Free PMC article. Review.
-
Characterization of a Fatty Acid Amide Hydrolase (FAAH) in Hirudo verbana.Res Sq [Preprint]. 2024 Apr 18:rs.3.rs-4271305. doi: 10.21203/rs.3.rs-4271305/v1. Res Sq. 2024. Update in: Neurochem Res. 2024 Nov;49(11):3015-3029. doi: 10.1007/s11064-024-04216-7. PMID: 38699363 Free PMC article. Updated. Preprint.
References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Siden-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195, 2000 - PubMed
-
- Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol 78: 17–37, 2006 - PubMed
-
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163: 463–468, 2003 - PMC - PubMed
-
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31: 617–630, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

