Late-expiratory activity: emergence and interactions with the respiratory CpG
- PMID: 20884764
- PMCID: PMC2997033
- DOI: 10.1152/jn.00334.2010
Late-expiratory activity: emergence and interactions with the respiratory CpG
Abstract
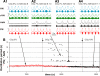
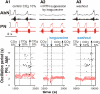
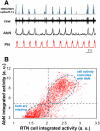
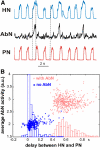
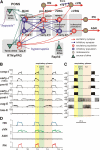
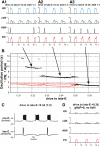
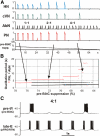
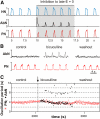
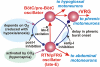
The respiratory rhythm and motor pattern are hypothesized to be generated by a brain stem respiratory network with a rhythmogenic core consisting of neural populations interacting within and between the pre-Bötzinger (pre-BötC) and Bötzinger (BötC) complexes and controlled by drives from other brain stem compartments. Our previous large-scale computational model reproduced the behavior of this network under many different conditions but did not consider neural oscillations that were proposed to emerge within the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) and drive preinspiratory (or late-expiratory, late-E) discharges in the abdominal motor output. Here we extend the analysis of our previously published data and consider new data on the generation of abdominal late-E activity as the basis for extending our computational model. The extended model incorporates an additional late-E population in RTN/pFRG, representing a source of late-E oscillatory activity. In the proposed model, under normal metabolic conditions, this RTN/pFRG oscillator is inhibited by BötC/pre-BötC circuits, and the late-E oscillations can be released by either hypercapnia-evoked activation of RTN/pFRG or by hypoxia-dependent suppression of RTN/pFRG inhibition by BötC/pre-BötC. The proposed interactions between BötC/pre-BötC and RTN/pFRG allow the model to reproduce several experimentally observed behaviors, including quantal acceleration of abdominal late-E oscillations with progressive hypercapnia and quantal slowing of phrenic activity with progressive suppression of pre-BötC excitability, as well as to predict a release of late-E oscillations by disinhibition of RTN/pFRG under normal conditions. The extended model proposes mechanistic explanations for the emergence of RTN/pFRG oscillations and their interaction with the brain stem respiratory network.
Figures




Similar articles
-
Interacting oscillations in neural control of breathing: modeling and qualitative analysis.J Comput Neurosci. 2011 Jun;30(3):607-32. doi: 10.1007/s10827-010-0281-0. Epub 2010 Oct 7. J Comput Neurosci. 2011. PMID: 20927576 Free PMC article.
-
Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats.J Physiol. 2017 Mar 15;595(6):2043-2064. doi: 10.1113/JP273335. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28004411 Free PMC article.
-
Interaction between the retrotrapezoid nucleus and the parafacial respiratory group to regulate active expiration and sympathetic activity in rats.Am J Physiol Lung Cell Mol Physiol. 2018 Nov 1;315(5):L891-L909. doi: 10.1152/ajplung.00011.2018. Epub 2018 Sep 6. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 30188747 Free PMC article.
-
Structural and functional architecture of respiratory networks in the mammalian brainstem.Philos Trans R Soc Lond B Biol Sci. 2009 Sep 12;364(1529):2577-87. doi: 10.1098/rstb.2009.0081. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19651658 Free PMC article. Review.
-
Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation.Prog Brain Res. 2007;165:201-20. doi: 10.1016/S0079-6123(06)65013-9. Prog Brain Res. 2007. PMID: 17925248 Free PMC article. Review.
Cited by
-
Modulation of hypercapnic respiratory response by cholinergic transmission in the commissural nucleus of the solitary tract.Pflugers Arch. 2020 Jan;472(1):49-60. doi: 10.1007/s00424-019-02341-9. Epub 2019 Dec 28. Pflugers Arch. 2020. PMID: 31884528
-
Reduced computational modelling of Kölliker-Fuse contributions to breathing patterns in Rett syndrome.J Physiol. 2019 May;597(10):2651-2672. doi: 10.1113/JP277592. Epub 2019 Apr 16. J Physiol. 2019. PMID: 30908648 Free PMC article.
-
Organization of the core respiratory network: Insights from optogenetic and modeling studies.PLoS Comput Biol. 2018 Apr 26;14(4):e1006148. doi: 10.1371/journal.pcbi.1006148. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29698394 Free PMC article.
-
Computational models for the study of heart-lung interactions in mammals.Wiley Interdiscip Rev Syst Biol Med. 2012 Mar-Apr;4(2):163-70. doi: 10.1002/wsbm.167. Epub 2011 Dec 2. Wiley Interdiscip Rev Syst Biol Med. 2012. PMID: 22140008 Free PMC article. Review.
-
Control of breathing by interacting pontine and pulmonary feedback loops.Front Neural Circuits. 2013 Feb 13;7:16. doi: 10.3389/fncir.2013.00016. eCollection 2013. Front Neural Circuits. 2013. PMID: 23408512 Free PMC article.
References
-
- Abu-Shaweesh JM, Dreshaj IA, Thomas AJ, Haxhiu MA, Strohl KP, Martin RJ. Changes in respiratory timing induced by hypercapnia in maturing rats. J Appl Physiol 87: 484–490, 1999 - PubMed
-
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res 128: 109–117, 1999 - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol 59: 583–634, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

