Loss of microRNAs in neural crest leads to cardiovascular syndromes resembling human congenital heart defects
- PMID: 20884876
- PMCID: PMC2988089
- DOI: 10.1161/ATVBAHA.110.213306
Loss of microRNAs in neural crest leads to cardiovascular syndromes resembling human congenital heart defects
Abstract
Objective: Congenital heart defects represent the most common human birth defects. Even though the genetic cause of these syndromes has been linked to candidate genes, the underlying molecular mechanisms are still largely unknown. Disturbance of neural crest cell (NCC) migration into the derivatives of the pharyngeal arches and pouches can account for many of the developmental defects. The goal of this study was to investigate the function of microRNA (miRNA) in NCCs and the cardiovascular system.
Methods and results: We deleted Dicer from the NCC lineage and showed that Dicer conditional mutants exhibit severe defects in multiple craniofacial and cardiovascular structures, many of which are observed in human neuro-craniofacial-cardiac syndrome patients. We found that cranial NCCs require Dicer for their survival and that deletion of Dicer led to massive cell death and complete loss of NCC-derived craniofacial structures. In contrast, Dicer and miRNAs were not essential for the survival of cardiac NCCs. However, the migration and patterning of these cells were impaired in Dicer knockout mice, resulting in a spectrum of cardiovascular abnormalities, including type B interrupted aortic arch, double-outlet right ventricle, and ventricular septal defect. We showed that Dicer loss of function was, at least in part, mediated by miRNA-21 (miR-21) and miRNA-181a (miR-181a), which in turn repressed the protein level of Sprouty 2, an inhibitor of Erk1/2 signaling.
Conclusions: Our results uncovered a central role for Dicer and miRNAs in NCC survival, migration, and patterning in craniofacial and cardiovascular development which, when mutated, lead to congenital neuro-craniofacial-cardiac defects.
Figures


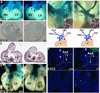
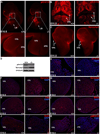
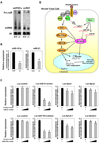
Similar articles
-
Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects.Dev Biol. 2013 Nov 15;383(2):239-52. doi: 10.1016/j.ydbio.2013.09.013. Epub 2013 Sep 18. Dev Biol. 2013. PMID: 24056078
-
Dicer activity in neural crest cells is essential for craniofacial organogenesis and pharyngeal arch artery morphogenesis.Mech Dev. 2011 Mar-Apr;128(3-4):200-7. doi: 10.1016/j.mod.2010.12.002. Epub 2011 Jan 21. Mech Dev. 2011. PMID: 21256960 Free PMC article.
-
mTOR acts as a pivotal signaling hub for neural crest cells during craniofacial development.PLoS Genet. 2018 Jul 5;14(7):e1007491. doi: 10.1371/journal.pgen.1007491. eCollection 2018 Jul. PLoS Genet. 2018. PMID: 29975682 Free PMC article.
-
Cranial neural crest cells on the move: their roles in craniofacial development.Am J Med Genet A. 2011 Feb;155A(2):270-9. doi: 10.1002/ajmg.a.33702. Epub 2010 Dec 10. Am J Med Genet A. 2011. PMID: 21271641 Free PMC article. Review.
-
Neural crest and cardiovascular development: a 20-year perspective.Birth Defects Res C Embryo Today. 2003 Feb;69(1):2-13. doi: 10.1002/bdrc.10002. Birth Defects Res C Embryo Today. 2003. PMID: 12768653 Review.
Cited by
-
microRNAs in cardiovascular development.J Mol Cell Cardiol. 2012 May;52(5):949-57. doi: 10.1016/j.yjmcc.2012.01.012. Epub 2012 Jan 24. J Mol Cell Cardiol. 2012. PMID: 22300733 Free PMC article. Review.
-
MicroRNA expression, target genes, and signaling pathways in infants with a ventricular septal defect.Mol Cell Biochem. 2018 Feb;439(1-2):171-187. doi: 10.1007/s11010-017-3146-2. Epub 2017 Aug 18. Mol Cell Biochem. 2018. PMID: 28822034 Clinical Trial.
-
Recommendations for Design, Execution, and Reporting of Studies on Experimental Thoracic Aortopathy in Preclinical Models.Arterioscler Thromb Vasc Biol. 2025 May;45(5):609-631. doi: 10.1161/ATVBAHA.124.320259. Epub 2025 Mar 13. Arterioscler Thromb Vasc Biol. 2025. PMID: 40079138 Review.
-
DiGeorge syndrome critical region 8 (DGCR8) protein-mediated microRNA biogenesis is essential for vascular smooth muscle cell development in mice.J Biol Chem. 2012 Jun 1;287(23):19018-28. doi: 10.1074/jbc.M112.351791. Epub 2012 Apr 17. J Biol Chem. 2012. PMID: 22511778 Free PMC article.
-
Life-long reduction in myomiR expression does not adversely affect skeletal muscle morphology.Sci Rep. 2019 Apr 2;9(1):5483. doi: 10.1038/s41598-019-41476-8. Sci Rep. 2019. PMID: 30940834 Free PMC article.
References
-
- Perez E, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge and velocardiofacial syndromes) Curr Opin Pediatr. 2002;14:678–683. - PubMed
-
- Emanuel BS, Shaikh TH. Segmental duplications: an 'expanding' role in genomic instability and disease. Nat Rev Genet. 2001;2:791–800. - PubMed
-
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

