The role of endoplasmic reticulum stress in the progression of atherosclerosis
- PMID: 20884885
- PMCID: PMC2951143
- DOI: 10.1161/CIRCRESAHA.110.224766
The role of endoplasmic reticulum stress in the progression of atherosclerosis
Abstract
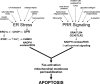
Prolonged activation of the endoplasmic reticulum (ER) stress pathway known as the unfolded protein response (UPR) can lead to cell pathology and subsequent tissue dysfunction. There is now ample evidence that the UPR is chronically activated in atherosclerotic lesional cells, particularly advanced lesional macrophages and endothelial cells. The stressors in advanced lesions that can lead to prolonged activation of the UPR include oxidative stress, oxysterols, and high levels of intracellular cholesterol and saturated fatty acids. Importantly, these arterial wall stressors may be especially prominent in the settings of obesity, insulin resistance, and diabetes, all of which promote the clinical progression of atherosclerosis. In the case of macrophages, prolonged ER stress triggers apoptosis, which in turn leads to plaque necrosis if the apoptotic cells are not rapidly cleared. ER stress-induced endothelial cell apoptosis may also contribute to plaque progression. Another potentially important proatherogenic effect of prolonged ER stress is activation of inflammatory pathways in macrophages and, perhaps in response to atheroprone shear stress, endothelial cells. Although exciting work over the last decade has begun to shed light on the mechanisms and in vivo relevance of ER stress-driven atherosclerosis, much more work is needed to fully understand this area and to enable an informed approach to therapeutic translation.
Figures

Similar articles
-
Mechanisms of ER stress-induced apoptosis in atherosclerosis.Arterioscler Thromb Vasc Biol. 2011 Dec;31(12):2792-7. doi: 10.1161/ATVBAHA.111.224881. Arterioscler Thromb Vasc Biol. 2011. PMID: 22096099 Free PMC article. Review.
-
Methods and models for monitoring UPR-associated macrophage death during advanced atherosclerosis.Methods Enzymol. 2011;489:277-96. doi: 10.1016/B978-0-12-385116-1.00016-9. Methods Enzymol. 2011. PMID: 21266236 Free PMC article.
-
The UPR in atherosclerosis.Semin Immunopathol. 2013 May;35(3):321-32. doi: 10.1007/s00281-013-0372-x. Epub 2013 Apr 4. Semin Immunopathol. 2013. PMID: 23553213 Free PMC article. Review.
-
Macrophage apoptosis in advanced atherosclerosis.Ann N Y Acad Sci. 2009 Sep;1173 Suppl 1(Suppl 1):E40-5. doi: 10.1111/j.1749-6632.2009.04957.x. Ann N Y Acad Sci. 2009. PMID: 19751413 Free PMC article.
-
Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target.Oxid Med Cell Longev. 2020 Sep 9;2020:9270107. doi: 10.1155/2020/9270107. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32963706 Free PMC article. Review.
Cited by
-
High-Density Lipoprotein Prevents Endoplasmic Reticulum Stress-Induced Downregulation of Liver LOX-1 Expression.PLoS One. 2015 Apr 29;10(4):e0124285. doi: 10.1371/journal.pone.0124285. eCollection 2015. PLoS One. 2015. PMID: 25923692 Free PMC article.
-
Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment.Diabetes. 2012 Oct;61(10):2609-20. doi: 10.2337/db11-1415. Epub 2012 Jun 29. Diabetes. 2012. PMID: 22751695 Free PMC article.
-
Serum Homocysteine and Vascular Calcification: Advances in Mechanisms, Related Diseases, and Nutrition.Korean J Fam Med. 2022 Sep;43(5):277-289. doi: 10.4082/kjfm.21.0227. Epub 2022 Sep 20. Korean J Fam Med. 2022. PMID: 36168899 Free PMC article.
-
Vascular ADAM17 as a Novel Therapeutic Target in Mediating Cardiovascular Hypertrophy and Perivascular Fibrosis Induced by Angiotensin II.Hypertension. 2016 Oct;68(4):949-955. doi: 10.1161/HYPERTENSIONAHA.116.07620. Epub 2016 Aug 1. Hypertension. 2016. PMID: 27480833 Free PMC article.
-
Amelioration of glucolipotoxicity-induced endoplasmic reticulum stress by a "chemical chaperone" in human THP-1 monocytes.Exp Diabetes Res. 2012;2012:356487. doi: 10.1155/2012/356487. Epub 2012 Apr 10. Exp Diabetes Res. 2012. PMID: 22550476 Free PMC article.
References
-
- Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. - PubMed
-
- NHLBI Morbidity and Mortality Chart Book. 2007
-
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

