Fluorescent labeling of tetracysteine-tagged proteins in intact cells
- PMID: 20885379
- PMCID: PMC3086663
- DOI: 10.1038/nprot.2010.129
Fluorescent labeling of tetracysteine-tagged proteins in intact cells
Abstract
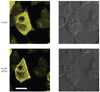
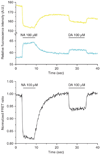
In this paper, we provide a general protocol for labeling proteins with the membrane-permeant fluorogenic biarsenical dye fluorescein arsenical hairpin binder-ethanedithiol (FlAsH-EDT₂). Generation of the tetracysteine-tagged protein construct by itself is not described, as this is a protein-specific process. This method allows site-selective labeling of proteins in living cells and has been applied to a wide variety of proteins and biological problems. We provide here a generally applicable labeling procedure and discuss the problems that can occur as well as general considerations that must be taken into account when designing and implementing the procedure. The method can even be applied to proteins with expression below 1 pmol mg⁻¹ of protein, such as G protein-coupled receptors, and it can be used to study the intracellular localization of proteins as well as functional interactions in fluorescence resonance energy transfer experiments. The labeling procedure using FlAsH-EDT₂ as described takes 2-3 h, depending on the number of samples to be processed.
Figures





References
-
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat. Methods. 2005;2:905–909. - PubMed
-
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. - PubMed
-
- Gautier A, et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 2008;15:128–136. - PubMed
-
- Gronemeyer T, Godin G, Johnsson K. Adding value to fusion proteins through covalent labelling. Curr. Opin. Biotechnol. 2005;16:453–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

