Novel role of KCNQ2/3 channels in regulating neuronal cell viability
- PMID: 20885443
- PMCID: PMC3017650
- DOI: 10.1038/cdd.2010.120
Novel role of KCNQ2/3 channels in regulating neuronal cell viability
Abstract
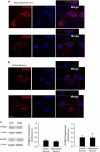
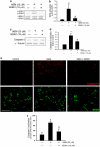
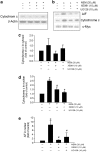
Overactivation of certain K(+) channels can mediate excessive K(+) efflux and intracellular K(+) depletion, which are early ionic events in apoptotic cascade. The present investigation examined a possible role of the KCNQ2/3 channel or M-channel (also named Kv7.2/7.3 channels) in the pro-apoptotic process. Whole-cell recordings detected much larger M-currents (212 ± 31 pA or 10.5 ± 1.5 pA/pF) in cultured hippocampal neurons than that in cultured cortical neurons (47 ± 21 pA or 2.4 ± 0.8 pA/pF). KCNQ2/3 channel openers N-ethylmaleimide (NEM) and flupirtine caused dose-dependent K(+) efflux, intracellular K(+) depletion, and cell death in hippocampal cultures, whereas little cell death was induced by NEM in cortical cultures. The NEM-induced cell death was antagonized by co-applied KCNQ channel inhibitor XE991 (10 μM), or by elevated extracellular K(+) concentration. Supporting a mediating role of KCNQ2/3 channels in apoptosis, expression of KCNQ2 or KCNQ2/3 channels in Chinese hamster ovary (CHO) cells initiated caspase-3 activation. Consistently, application of NEM (20 μM, 8 h) in hippocampal cultures similarly caused caspase-3 activation assessed by immunocytochemical staining and western blotting. NEM increased the expression of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), induced mitochondria membrane depolarization, cytochrome c release, formation of apoptosome complex, and apoptosis-inducing factor (AIF) translocation into nuclear. All these events were attenuated by blocking KCNQ2/3 channels. These findings provide novel evidence that KCNQ2/3 channels could be an important regulator in neuronal apoptosis.
Figures







Similar articles
-
Potential role of KCNQ/M-channels in regulating neuronal differentiation in mouse hippocampal and embryonic stem cell-derived neuronal cultures.Exp Neurol. 2011 Jun;229(2):471-83. doi: 10.1016/j.expneurol.2011.03.018. Epub 2011 Apr 2. Exp Neurol. 2011. PMID: 21466805 Free PMC article.
-
Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent.J Neurosci. 2004 Jun 2;24(22):5079-90. doi: 10.1523/JNEUROSCI.0882-04.2004. J Neurosci. 2004. PMID: 15175377 Free PMC article.
-
Suppression of KV7/KCNQ potassium channel enhances neuronal differentiation of PC12 cells.Neuroscience. 2016 Oct 1;333:356-67. doi: 10.1016/j.neuroscience.2016.07.024. Epub 2016 Jul 20. Neuroscience. 2016. PMID: 27450567
-
The KCNQ2/3 selective channel opener ICA-27243 binds to a novel voltage-sensor domain site.Neurosci Lett. 2009 Nov 13;465(2):138-42. doi: 10.1016/j.neulet.2009.08.071. Epub 2009 Sep 3. Neurosci Lett. 2009. PMID: 19733209
-
Made for "anchorin": Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons.Semin Cell Dev Biol. 2011 Apr;22(2):185-92. doi: 10.1016/j.semcdb.2010.10.001. Epub 2010 Oct 19. Semin Cell Dev Biol. 2011. PMID: 20940059 Free PMC article. Review.
Cited by
-
Calmodulin regulates KCNQ2 function in epilepsy.Am J Transl Res. 2016 Dec 15;8(12):5610-5618. eCollection 2016. Am J Transl Res. 2016. PMID: 28078031 Free PMC article.
-
Ionic regulation of cell volume changes and cell death after ischemic stroke.Transl Stroke Res. 2014 Feb;5(1):17-27. doi: 10.1007/s12975-013-0314-x. Epub 2013 Dec 7. Transl Stroke Res. 2014. PMID: 24323733 Free PMC article. Review.
-
Identification of common genetic variants in KCNQ family genes associated with gastric cancer survival in a Chinese population.J Biomed Res. 2024 May 29;39(1):76-86. doi: 10.7555/JBR.38.20240040. J Biomed Res. 2024. PMID: 38807370 Free PMC article.
-
Genetic Polymorphisms Associated with Hearing Threshold Shift in Subjects during First Encounter with Occupational Impulse Noise.PLoS One. 2015 Jun 29;10(6):e0130827. doi: 10.1371/journal.pone.0130827. eCollection 2015. PLoS One. 2015. PMID: 26121033 Free PMC article.
-
Characteristics and molecular basis of celecoxib modulation on K(v)7 potassium channels.Br J Pharmacol. 2011 Nov;164(6):1722-37. doi: 10.1111/j.1476-5381.2011.01483.x. Br J Pharmacol. 2011. PMID: 21564087 Free PMC article.
References
-
- Burg ED, Remillard CV, Yuan JX. K+ channels in apoptosis. J Membr Biol. 2006;209:3–20. - PubMed
-
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. - PubMed
-
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, et al. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

