Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3-kinase
- PMID: 20885446
- PMCID: PMC3132006
- DOI: 10.1038/cdd.2010.118
Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3-kinase
Abstract
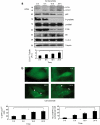
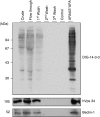
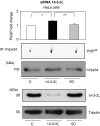
14-3-3s are binding proteins with survival functions in cells by interaction with proteins involved in the regulation of cell fate. The role of 14-3-3 during autophagy was investigated, thus, a forced expression of 14-3-3ζ reduces C2-ceramide-induced autophagy, whereas depletion of 14-3-3ζ promotes autophagy. The 14-3-3 role in autophagyc-related proteins was also investigated. The human vacuolar protein sorting 34 (hVps34), the class III phosphatidylinositol-3-kinase mediates multiple vesicle-trafficking processes such as endocytosis and autophagy, its activation being a requirement for autophagy initiation. Using chromatography techniques, hVps34 were eluted from a 14-3-3 affinity column, showing also a direct interaction with 14-3-3 proteins under physiological condition. Further analysis suggests that hVps34/14-3-3 association is a phorbol-12-myristate-13-acetate-dependent phosphorylated mechanism promoting a strong inhibition of the hVps34 lipid kinase activity, proteins kinase C being the likely kinase involved in phosphorylation and 14-3-3 binding of hVps34 under physiological conditions. Meanwhile, stimulation of autophagy leads to the dissociation of the 14-3-3/hVps34 complex enhancing hVps34 lipid kinase activity. Forced expression of 14-3-3ζ reduces hVps34 kinase activity and depletion of 14-3-3ζ promotes upregulation of this activity. In this study, 14-3-3ζ proteins are shown as a negative regulator of autophagy through regulation of a key component of early stages of the autophagy pathway, such as hVps34.
Figures


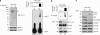




References
-
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev. 2005;5:886–897. - PubMed
-
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. - PubMed
-
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

