Myotonic dystrophies 1 and 2: complex diseases with complex mechanisms
- PMID: 20885816
- PMCID: PMC2874224
- DOI: 10.2174/138920210790886844
Myotonic dystrophies 1 and 2: complex diseases with complex mechanisms
Abstract
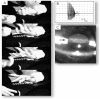
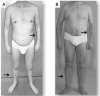
Two multi-system disorders, Myotonic Dystrophies type 1 and type 2 (DM1 and DM2), are complex neuromuscular diseases caused by an accumulation of expanded, non-coding RNAs, containing repetitive CUG and CCUG elements. Similarities of these mutations suggest similar mechanisms for both diseases. The expanded CUGn and CCUGn RNAs mainly target two RNA binding proteins, MBNL1 and CUGBP1, elevating levels of CUGBP1 and reducing levels of MBNL1. These alterations change processing of RNAs that are regulated by these proteins. Whereas overall toxicity of CUGn/CCUGn RNAs on RNA homeostasis in DM cells has been proven, the mechanisms which make these RNAs toxic remain illusive. A current view is that the toxicity of RNA CUGn and CCUGn is associated exclusively with global mis-splicing in DM patients. However, a growing number of new findings show that the expansion of CUGn and CCUGn RNAs mis-regulates several additional pathways in nuclei and cytoplasm of cells from patients with DM1 and DM2. The purpose of this review is to discuss the similarities and differences in the clinical presentation and molecular genetics of both diseases. We will also discuss the complexity of the molecular abnormalities in DM1 and DM2 caused by CUG and CCUG repeats and will summarize the outcomes of the toxicity of CUG and CCUG repeats.
Figures





References
-
- Steinert H. Myopathologische Beiträge 1. Über das klinische und anatomische Bild des Muskelschwunds der Myotoniker. Dtsch. Zeitschr. Nervenheilk. 1909;37:58–104.
-
- Curschmann H. Über familiäre atrophische Myotonie. Dtsch. Zeitschr Nervenheilk. 1912;45:161–202.
-
- Harper PS. Myotonic Dystrophy. 3rd. Vol. 37. Philadelphia: W.B. Saunders; 2001. Major Problems in Neurology.
-
- Fleischer B. Über myotonische Dystrophie mit Katarakt. Graefes. Arch. Klein. Ophthalmol. 1918;96:91–133.
Grants and funding
LinkOut - more resources
Full Text Sources
