Immune response following vaccination against Salmonella Enteritidis using 2 commercial bacterins in laying hens
- PMID: 20885842
- PMCID: PMC2896799
Immune response following vaccination against Salmonella Enteritidis using 2 commercial bacterins in laying hens
Abstract
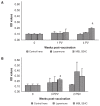
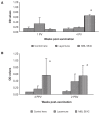
The humoral and cell-mediated immune (CMI) response to 2 commercial killed Salmonella Enteritidis (SE) vaccines (Layermune and MBL SE4C) was evaluated in laying hens. Layers were distributed in 2 experimental groups. The first received a single immunization at 16 wk of age, while the second experimental group was immunized at 12 wk of age and again at 18 wk of age. Serum immunoglobulin (Ig)G antibodies were measured using a commercial SE ELISA kit and showed persistent levels from 3 to 32 and 34 wk post-vaccination. The vaccination protocol using 2 immunizations showed a higher seroconversion level than the single vaccination. However, our results for bacterial intracellular survival indicated that IgG titers were not linked with bacterial killing. Local IgA production was measured in the intestines and oviducts with an in-house SE whole cell antigen ELISA. Only the MBL SE4C vaccine elicited IgA antibody production when tested on intestine and oviduct mucosal secretions, 3-weeks post-vaccination in both immunization protocol groups. To evaluate the CMI response, the splenic T-cells and B-cells populations were analyzed using flow cytometry. The CD3/B-cell ratio decreased 3 wk after the second immunization in the twice vaccinated Layermune group due to an increase in B-cells.
La réponse immunitaire humorale et à médiation cellulaire suite à l’administration de vaccins tués contre Salmonella Enteritidis (SE) (Layermune ou MBL SE4C) a été évaluée chez des poules pondeuses. Les poules ont été séparées en deux groupes, soit le premier recevant une seule immunisation à l’âge de 16 semaines, et le second groupe recevant deux immunisations à l’âge de 12 et 18 semaines. Les anticorps sériques (Ig)G mesurés à l’aide d’une trousse ELISA commerciale pour SE ont démontré des niveaux persistant de la 3e aux 32e et 34e semaines post-vaccination. Le protocole vaccinal avec 2 immunisations a démontré la séroconversion la plus élevée comparativement au protocole avec une seule immunisation. Cependant, nos résultats concernant la survie intracellulaire bactérienne indiquent que ces titres IgG ne sont pas associés à la mort bactérienne. La production locale d’IgA a été mesurée dans les sécrétions mucosales de l’intestin et de l’oviducte avec un test ELISA maison. Seul le vaccin MBL SE4C a stimulé la production d’IgA locaux, 3 semaines post-vaccination et ce, avec les deux protocoles vaccinaux. Afin d’évaluer la réponse à médiation cellulaire, les populations de cellules spléniques T et B ont été analysées à l’aide de la cytométrie en flux. Le ratio CD3/cellules B a diminué 3 semaines après la seconde immunisation dans le groupe vaccinés deux fois avec le vaccin Layermune et ce, suite à une augmentation du nombre de cellules B.
(Traduit par les auteurs)
Figures


Similar articles
-
Comparison of a live attenuated Salmonella Enteritidis vaccine candidate secreting Escherichia coli heat-labile enterotoxin B subunit with a commercial vaccine for efficacy of protection against internal egg contamination by Salmonella in hens.Can J Vet Res. 2015 Jul;79(3):235-40. Can J Vet Res. 2015. PMID: 26130857 Free PMC article. Clinical Trial.
-
Induction of humoral immune response and protective immunity in chickens against Salmonella enteritidis after a single dose of killed bacterium-loaded microspheres.Avian Dis. 2001 Oct-Dec;45(4):797-806. Avian Dis. 2001. PMID: 11785884
-
Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-gamma production.Comp Immunol Microbiol Infect Dis. 2004 Jul;27(4):255-72. doi: 10.1016/j.cimid.2003.12.001. Comp Immunol Microbiol Infect Dis. 2004. PMID: 15178000
-
Humoral and cellular immune response generated by different vaccine programs before and after Salmonella Enteritidis challenge in chickens.Vaccine. 2012 Dec 14;30(52):7637-43. doi: 10.1016/j.vaccine.2012.10.020. Epub 2012 Oct 18. Vaccine. 2012. PMID: 23085366
-
[Protection against Salmonella via immunization with recombinant lactic acid bacteria].Nihon Rinsho. 2012 Aug;70(8):1293-7. Nihon Rinsho. 2012. PMID: 22894061 Review. Japanese.
Cited by
-
Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens.Int J Nanomedicine. 2020 Feb 3;15:761-777. doi: 10.2147/IJN.S238445. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32099364 Free PMC article.
-
Local Inflammatory and Systemic Antibody Responses Initiated by a First Intradermal Administration of Autogenous Salmonella-Killed Vaccines and Their Components in Pullets.Vaccines (Basel). 2024 Oct 11;12(10):1159. doi: 10.3390/vaccines12101159. Vaccines (Basel). 2024. PMID: 39460325 Free PMC article.
-
Comparison of a live attenuated Salmonella Enteritidis vaccine candidate secreting Escherichia coli heat-labile enterotoxin B subunit with a commercial vaccine for efficacy of protection against internal egg contamination by Salmonella in hens.Can J Vet Res. 2015 Jul;79(3):235-40. Can J Vet Res. 2015. PMID: 26130857 Free PMC article. Clinical Trial.
-
Evaluation of the Safety and Protection Efficacy of spiC and nmpC or rfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis.Vaccines (Basel). 2019 Nov 30;7(4):202. doi: 10.3390/vaccines7040202. Vaccines (Basel). 2019. PMID: 31801257 Free PMC article.
-
Impact of Dietary Galacto-Oligosaccharide (GOS) on Chicken's Gut Microbiota, Mucosal Gene Expression, and Salmonella Colonization.Front Vet Sci. 2017 Nov 13;4:192. doi: 10.3389/fvets.2017.00192. eCollection 2017. Front Vet Sci. 2017. PMID: 29181381 Free PMC article.
References
-
- Methner UDR, Reiche R, Böhland K. Occurence of Salmonellae in laying hens in different housing systems and inferences for control. Berl Munch Tierarztl Wochenschr. 2006;119:467–473. - PubMed
-
- Davison S, Benson CE, Henzler DJ, Eckroade RJ. Field Observations with Salmonella Enteritidis Bacterins. Avian Dis. 1999;43:664–669. - PubMed
-
- Woodward MJ, Gettinby G, Breslin MF, Corkish JD, Houghton S. The efficacy of Salenvac, a Salmonella enterica subsp. enterica serotype Enteritidis iron-restricted bacterin vaccine, in laying chickens. Avian Pathol. 2002;31:383–392. - PubMed
-
- Piao Z, Toyota-Hanatani Y, Ohta H, Sasai K, Tani H, Baba E. Effects of Salmonella enterica subsp. enterica serovar Enteritidis vaccination in layer hens subjected to S. Enteritidis challenge and various feed withdrawal regimens. Vet Microbiol. 2007;125:111–119. - PubMed
-
- Babu UDR, Okamura M, Lillehoj HS, et al. Salmonella Enteritidis clearance and immune responses in chickens following Salmonella vaccination and challenge. Vet Immunol and Immunopathol. 2004;101:251–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
