The regulation of organ size in Drosophila: physiology, plasticity, patterning and physical force
- PMID: 20885854
- PMCID: PMC2901811
- DOI: 10.4161/org.6.2.10375
The regulation of organ size in Drosophila: physiology, plasticity, patterning and physical force
Abstract
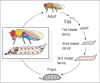
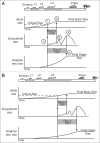
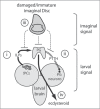
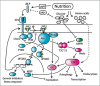
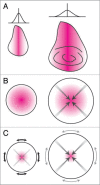
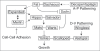
The correct regulation of organ size is a fundamental developmental process, the failure of which can compromise organ function and organismal integrity. Consequently, the mechanisms that regulate organ size have been subject to intense research. This research has highlighted four classes of mechanism that are involved in organ size regulation: physiology, plasticity, patterning and physical force. Nevertheless, how these mechanisms are integrated and converge on the cellular process that regulate organ growth is unknown. One group of animals where this integration is beginning to be achieved is in the insects. Here, I review the different mechanisms that regulate organ size in insects, and describe our current understanding of how these mechanisms interact. The genes and hormones involved are remarkably conserved in all animals, so these studies in insects provide a precedent for future research on organ size regulation in mammals.
Keywords: adhesion; hormone; hypoplasia; imaginal disc; insect; insulin; morphogens; size regulation.
Figures
References
-
- Nijhout HF. Physiological control of molting in insects. Am Zool. 1981;21:631–640.
-
- Riddiford LM. Hormones and Drosophila development. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Press; 1993. pp. 899–939.
-
- Truman JW, Riddiford LM. Physiology of insect rhythms 3. The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol. 1974;60:371–382. - PubMed
-
- Kawasaki H. Transition from larva to pupa: morphogenesis, cell proliferation and protein synthesis in Bombyx wing disc. Inv Repro Devel. 1998;34:101–108.
-
- Ohtaki T, Yamanaka F, Sakurai S. Differential timing of pupal commitment in various tissues of the silkworm, Bombyx mori. J Insect Physiol. 1986;32:635.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
